
STABILITY-INDICATING HPLC METHOD FOR THE DETERMINATION OF RELATED SUBSTANCES IN LANSOPRAZOLE INTERMEDIATE
Dr. Balaji Nagarajan 1![]()
![]() ,
Dr. Gunasekar Manoharan 2
,
Dr. Gunasekar Manoharan 2![]()
![]()
1,2 New Jersey Bioscience
Centre, 685, North Brunswick, New Jersey, 08902, USA
|
|
ABSTRACT |
||
|
A novel, reversed-phase liquid chromatographic method was developed and validated for the determination of related substances in lansoprazole intermediate. Symmetric peak shape was on a C18 stationary phase with the dimensions of 250 mm column length, 4.6 mm as internal diameter, 5 microns particles with an economical and straightforward mass-compatible mobile phase combination of formic acid/triethylamine and acetonitrile delivered in gradient mode at a flow rate of 1.0 mL/min at 260 nm. The resolution between lansoprazole intermediate (LAN20) and its impurities (LAN20-I & LAN20-II) in the developed method was more than 2.0, indicating a significant separation. Regression analysis shows a correlation coefficient greater than 0.999 for lansoprazole intermediate and its related substances. The detection and quantitation limits of lansoprazole intermediate and its impurities are 0.01% and 0.005%. This method indicates that the recovery at different levels is 90 to 110% accurate. The test solution was stable in the diluent for 48 h and subjected to stress conditions. The mass balance was close to 99.5%. |
|||
|
Received 02 April 2022 Accepted 05 May 2022 Published 23 May 2022 Corresponding Author Balaji
Nagarajan, DOI 10.29121/IJOEST.v6.i3.2022.329 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2022 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Lansoprazole
Intermediate, HPLC, Validation, Impurities, Stress Study |
|||
1. INTRODUCTION
Lansoprazole is used to treat and prevent stomach and intestinal ulcers, erosive esophagitis (damage to the esophagus from stomach acid), and other conditions involving excessive stomach acid such as Zollinger-Ellison syndrome. Lansoprazole intermediate is the key raw material for the synthesis of lansoprazole drug substance. The purity of lansoprazole intermediate determines the quality of lansoprazole drug substance with high yield in the synthetic process during the manufacturing WIPO (2022), Chorghade (2006). Its molecular formula is C9H10Cl2F3NO. LAN20 is a white to tan powder. It is highly sensitive to light and moisture.
This study describes a simple, sensitive, and cost-effective mass-compatible mobile phase method for the quantitation of LAN20. The effort includes the method development and validation as per ICH guidelines Blvd and Diego (2014). Hitherto, there is no article for the quantification and determination of LAN20 and its impurities. This research study is a novel and sensitive method for the lansoprazole intermediate using HPLC and using this work, the packaging integrity could be defined.
2. MATERIALS AND METHODS
2.1. MATERIALS
Sigma Aldrich, USA, supplied lansoprazole intermediate and used it as a reference standard with the help of structural elucidation techniques. The impurities of lansoprazole intermediate have been gifted by Techno research laboratory, Mumbai, India. Formic acid, triethylamine, HPLC grade acetonitrile from Merck, Darmstadt, Germany. Millipore water purification system generated the USP purified water.
The HPLC system was an Agilent 1200 quaternary pump, an
autosampler, and a diode array detector. The chromatographic output signal was
monitored and processed by Empower software on an Intel Core i5 computer
(Dell). Hydrolytic studies have been conducted in a water bath with
controllers, Julabo, Seelbach, Germany. Stability studies were carried out in a
humidity chamber (Thermo lab humidity chamber, India), and light-sensitive
studies were conducted in a photostability chamber (Sanyo photostability
chamber, Leicestershire, UK). Thermal stability studies were performed in a dry
air oven (MACK Pharmatech, Hyderabad, India). The structure of the lansoprazole
intermediate and its impurities are given in Figure 1
Figure 1
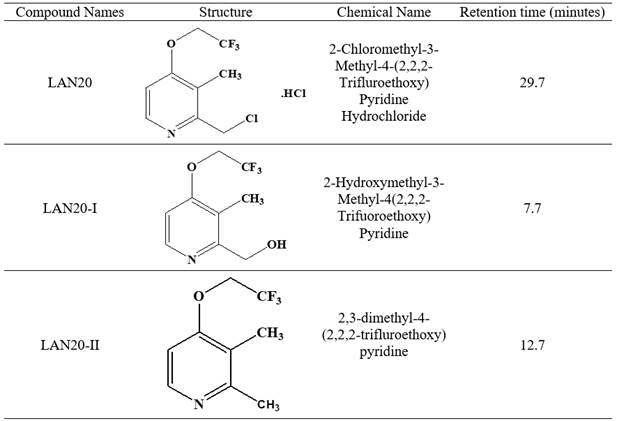
|
Figure 1 Structure of Lansoprazole intermediate and its impurities |
3. METHODS
3.1. CHROMATOGRAPHIC CONDITIONS
The wide bore column of Zorbax Eclipse XDB-C18 with a length of 250 mm, an internal diameter of 4.6 mm, and a 5.0 µm particle size has been manufactured and supplied by Agilent Technologies, USA, was used to determine lansoprazole intermediate and its impurities. The mobile phase A solution has prepared by adding 1.0 mL of formic acid in 1000 mL of water and acetonitrile as mobile phase B solution. The gradient mode was selected for this study and the composition of mobile phase B solution are 0.0/10, 10.0/10, 50.0/90, 51.0/10, and 60.0/10. The method flow rate was 1.0 mL/min. Monitored the column temperature throughout the analysis at 45°C and monitored the detection was at a wavelength of 260 nm. The injection volume was 20 µL. Mixed about 900 mL of mobile phase A and 100 mL of acetonitrile in a 1L bottle and marked as diluent.
Preparation of stock solutions, system suitability, and
sample
Accurately weighed and transferred 5 mg each of LAN20, LAN20-I, and LAN20-II into a 50 mL volumetric flask. Dissolved in 20 mL of diluent and made up to the volume with diluent and marked as A. Pipetted out 5.0 mL of the A solution and transferred into a 50 mL volumetric flask, made up to the volume with diluent and marked as B. This solution is considered as standard solution with the concentration of 10 µg/ mL for LAN20, LAN20-I, LAN20-II and used as system suitability for related substances determinations.
4. ANALYTICAL METHOD DEVELOPMENT
Lansoprazole intermediate and its impurities had UV maxima at around 260 nm; monitored detection at 260 nm for the method development. The primary concern was obtaining the resolution between the analyte and its impurities peak symmetry to develop a selective and sensitive method.
By modifying the column oven temperature to 45°C, the LAN20 and its impurities peak symmetry has significantly improved, which has impacted the USP Tailing factor to 1.2. The resolution between LAN20 and known peaks has been substantially enhanced by altering acetonitrile's composition. The results were satisfactory by using these chromatographic conditions. Buffer pH and % acetonitrile did not play a significant role in peak shape for Lansoprazole intermediate. This study has executed the analysis with different batches of bulk drug intermediate samples (n = 3). Results were within specification limits.
4.1. STATISTICAL METHOD
Using the Minitab 17 software, executed the robustness study with four number factors viz., flow, column oven temperature, mobile phase organic ratio, and wavelength.
5. RESULTS AND DISCUSSION
5.1. ANALYTICAL METHOD VALIDATION
The chromatographic method was developed and validated for selectivity, linearity, range, precision, accuracy, sensitivity, robustness, and system suitability.
5.2. SPECIFICITY
All stress decomposition studies were at an initial drug concentration of 2000 µg/mL. Forced degradation studies were performed on LAN20 to indicate the stability-indicating property and specificity of the proposed method. The representation of the standard chromatogram is illustrated in Figure 2.
Figure 2
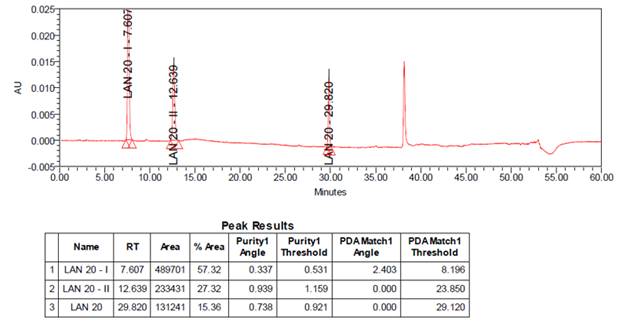
|
Figure 2 Chromatogram of LAN20 and impurities standard |
5.3. RESULTS OF FORCED DEGRADATION STUDIES
Stress studies were on LAN20 under different stress conditions. The research study exposed the LAN20 to acid, base hydrolysis, oxidation under reflux conditions. LAN20 showed significant degradation towards the acid/base hydrolysis treatment of 0.1 N NaOH (24 h reflux at 80°C) and 0.1 N HCl (24 h reflux at 80°C) and the oxidative stress of the compound has performed with 3% hydrogen peroxide at room temperature for 24 h. LAN20 showed sensitive towards the treatment of hydrogen peroxide, acid, and base. No degradation was observed when the LAN20 was exposed to water at 60°C for 7 days and found to be very stable toward the heat. The LAN20 was highly sensitive to the effect of photolysis. Significant degradation was observed when the LAN20 powder was exposed to light for a total coverage of 1200000 lux hours and an integrated near ultraviolet energy of 200-Watt hours/square meter ICH Harmonised (1996) in a photostability chamber. The drug was stable to the effect of temperature. Peak purity results for stressed LAN20 samples, derived from the PDA detector (the purity angle within the purity threshold limit), confirm that the LAN20 peak was homogeneous and pure. No degradation product peaks were observed after 5 min in the extended run time of 25 min for all the LAN20 stressed samples. Assay studies were executed for stress samples against a qualified reference standard. The mass balance (% assay + % of impurities + % of degradation products) of stressed samples was close to 99.5%, confirming the stability-indicating power of the developed method.
5.4. LINEARITY AND RANGE
The linearity study has evaluated by determining nine concentration levels from LOQ to 200% of LAN20 and its impurities. The correlation coefficient obtained for LAN20-I, LAN20-II and LAN20 were 0.9999, and tabulated linearity results are in Table 1. The LAN20 and its impurities linearity graph demonstrated in Figure 3.
Table.1
|
Table 1 Compilation of concentrations and area responses for LAN20 and its impurities |
|||
|
Concentration (%) |
Area Response |
||
|
LAN20 |
LAN20-I |
LAN20-II |
|
|
30 |
39370 |
146910 |
70092 |
|
40 |
52495 |
195880 |
93372 |
|
80 |
104990 |
391768 |
186745 |
|
100 |
131241 |
489701 |
233481 |
|
120 |
157489 |
587649 |
280117 |
|
160 |
209986 |
783542 |
373490 |
|
200 |
262482 |
979462 |
466862 |
|
Slope |
131242 |
489731 |
233410 |
|
Intercept |
-2.4 |
-17.6 |
38.4 |
|
Correlation coefficient |
1 |
1 |
1 |
Figure 3
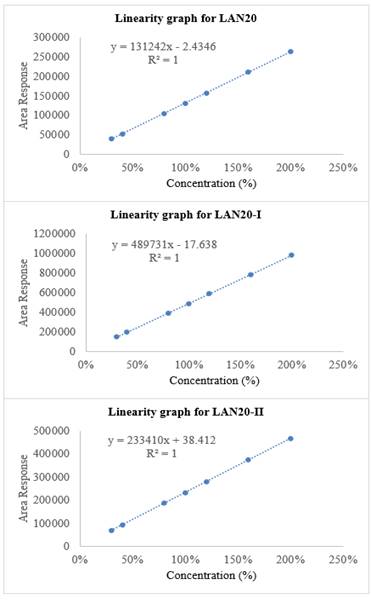
|
Figure 3 Linearity graph for LAN20, LAN20-I and LAN20-II |
Precision
The precision was checked by injecting six individual preparations of (10 µg/mL) LAN20 and its impurities. The % RSD for the percentage of LAN20 and its impurities were below 0.4%. The % RSD was within 0.8% in the intermediate precision. The different analysts and columns evaluated the intermediate precision of the method, and by using another instrument, % RSDs were within 1.8%, confirming the ruggedness of the method.
Sensitivity
Determined the sensitivity by establishing the LOD (3:1) and LOQ (10:1) for LAN20 and its impurities estimated by dividing the height of the peak and baseline noise by injecting a series of dilute solutions with known concentrations. The limit of detection and quantification for LAN20 and its impurities were 0.01% and 0.03%, respectively. The precision study at the LOQ level with six replicate injections of LOQ solution and the % RSD for the area was within 1.1% and found that the method was susceptible.
Accuracy
In the method validation study, the recovery parameter is essential to determine the method capability and performed in triplicate preparations at 50, 100, and 150% of the LAN20 specification limits (0.5%). The percentage of recovery for LAN20 and its impurities was between 95-105%, indicating that the method was suitable for determining LAN20 and its impurities.
Robustness
The robustness study was performed using the Minitab 17 software using the four number factors: flow, column oven temperature, mobile phase organic ratio, and wavelength. The resolution between the LAN20 and its impurities peaks by changing experimental conditions is in Table 2. The developed and validated method has a flow rate of 1.0 mL/min. To study the consequence of flow rate on the resolution, 0.1 units changed it from 1.1 to 0.9 mL/min. The result of column temperature on the resolution was studied at 40°C and 50°C instead of 45 °C. To check the effect of an organic ratio of 10:90 (%v/v), it was transformed from 9 to 11 (%v/v). The impact of the detection wavelength, 260 nm, changed it from 258 nm to 262 nm. In all the deliberate varied chromatographic conditions (flow rate, column oven temperature, and detection wavelength), the resolution between LAN20 and its impurities was more significant than 1.5, illustrating the method’s robustness.
Table.2
|
Table 2 Design of experiments for robustness study |
||||
|
Experiment |
Flow (mL/min) |
Column oven temperature (°C) |
Acetonitrile (% v/v) |
Wavelength (nm) |
|
1 |
0.9 |
50 |
9 |
258 |
|
2 |
1.1 |
50 |
11 |
262 |
|
3 |
1.1 |
50 |
11 |
262 |
|
4 |
0.9 |
40 |
11 |
262 |
|
5 |
1.1 |
40 |
11 |
262 |
|
6 |
1.1 |
40 |
11 |
258 |
|
7 |
1.1 |
50 |
9 |
258 |
|
8 |
0.9 |
50 |
11 |
258 |
|
9 |
0.9 |
50 |
11 |
258 |
|
10 |
1.1 |
40 |
11 |
258 |
|
11 |
0.9 |
50 |
11 |
262 |
|
12 |
0.9 |
40 |
9 |
258 |
|
13 |
0.9 |
40 |
9 |
258 |
|
14 |
0.9 |
50 |
9 |
262 |
|
15 |
0.9 |
40 |
11 |
262 |
|
16 |
1.1 |
50 |
11 |
258 |
Solution Stability
The solution stability of LAN20 and its impurities were carried out by leaving the sample solution, which was prepared in a volumetric flask and kept at room temperature for 48 hours. The mobile phase stability was performed using freshly prepared sample solutions against reference standard solutions at 48 hours. The %RSD of LAN20 and its impurities during solution stability and mobile phase stability experiments was 1.1%. There was no change observed in the content of LAN20 and its impurities during solution stability and mobile phase stability experiments. The data confirms that standard and sample solutions were stable for up to 48 hours.
6. CONCLUSION
A new, sensitive, and stability indicating HPLC method was successfully developed to quantify LAN20 and its impurities in bulk intermediates. The technique was accurate and precise, with excellent and consistent recoveries. The validated method may be used for the routine analysis of the determination of LAN20 and its impurities from the bulk drug, pharmaceutical preparation, and other quality control samples of product development.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
Blvd, S.V. and Diego, S.G.S (2014). ICH Harmonised tripartite guideline-Validation of analytical procedures: text and methodologyQ2(R1).
Chorghade, M. S. (2006). Drug Discovery and Development, Volume 1: Drug Discovery. 201. https://doi.org/10.1002/0471780103
Gangula, S. Elati, R.C. Neredla, A. Baddam, S.R. Neelam, U. Bandichhor, R. Dongamanti, A. (2010). An improved process for the production of lansoprazole : investigation of key parameters that influence the water content in final API. Org. Process Res. Dev, 14(1), 229- 233. https://doi.org/10.1021/op900258b
Hirschowitz, B.I. Mohnen, J. Shaw, S. (1996). Long-term treatment with lansoprazole for patients with Zollinger-Ellison syndrome. Aliment. Pharmacol. Ther. 10 (4). https://doi.org/10.1046/j.1365-2036.1996.10152000.x
ICH Harmonised (1996). Guideline-Stability testing: Photostability testing of new drug substances and products Q1B.
Lansoprazole Summary (2020). Lansoprazole - Drug Usage Statistics. ClinCalc.
Pharmaceuticals, M. (2016). Lansoprazole capsule, delayed release pellets.
WIPO (2022). PATENTSCOPE, Wockhardt limited, Maharashtra, India.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2022. All Rights Reserved.