
Investigation of Potassium Bromate in Bread from Bakeries in Ukanafun Community of Akwa Ibom State, Nigeria
Ubong I. Etukudo 1, Emmanuel J. Ekott
2![]() , Esther I. Ukpanah 1
, Esther I. Ukpanah 1
1 Department of Chemistry, Heritage
Polytechnic, Eket, Nigeria
|
|
ABSTRACT |
||
|
Potassium
bromate (KBrO3) is a dough improver used in baking bread to
increase bread size and profit. However, the additive has been banned from
use due to its adverse effects on human health. Despite its ban, some bakers,
especially in rural communities continue to use it. The ingestion of
potassium bromate is linked to a number of clinical
problems including carcinogenicity, hepatotoxicity and neurotoxicity. Bread
samples from five bakeries in rural communities in Akwa Ibom State, Nigeria
were randomly obtained and assessed for their levels of potassium bromate
using spectrophotometric method. The absorbance of the standards and that of
the samples were taken at 540 nm in the visible region of the
spectrophotometer, then converted to concentrations with reference to a
calibration curve constructed from pure potassium bromate sample solutions.
All the bread samples contained potassium bromate and concentrations in the
bread samples ranged from 5.16 – 8.50 mg/Kg. The values are beyond the
allowed permissible level set by most international health standards that
permit the use of the additive. The concentrations are higher than the
permissible level of 0.02 mg/Kg set in Nigeria by National Agency for Food
and Drug Administration and Control (NAFDAC). The ban should be enforced
further especially in bakeries sited in rural communities in
order to properly safeguard the health of consumers. |
|||
|
Received 01 January 2024 Accepted 29 January 2024 Published 15 February 2024 Corresponding Author Emmanuel
J. Ekott, eekott@yahoo.com DOI 10.29121/IJOEST.v8.i1.2024.553 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2024 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Bread,
Potassium Bromate, Bakeries, Additives, Health |
|||
1. INTRODUCTION
Bread is an important ‘ready to eat’ food in Nigeria. It is usually made with several ingredients that improves its quality and palatability. Some of the basic ingredients are flour, salt, sugar, flavors and at least a flour-improver such as potassium bromate Emeje et al. (2015). Naturally, flour has an improved baking properties if it is left to ‘age’ for a couple of months Vicki (2019). However, to meet up with the high demand for bread, the aging processes are forced using chemicals additives. During bread making, flour-improver or bread-improvers are added for firmness of mixing, ease molding, increase loaf size, texture and to maximize profit. However, some flour-improvers are unsafe due to their deleterious impacts on health Oloyede & Sunmoru (2009). Potassium bromate or simply called bromate has been a flour improver of choice for flour millers and bread bakers in Nigeria probably because it is inexpensive and effective throughout the fermentation and baking process that positively affect the structural and rheological properties of the dough Ekop et al. (2008).
Bromate has adverse effect on the health of consumers. Sai & Takagi (1991) reported that the chemical degrades nutrients in bread, thus more quantities of vitamins and other nutrients would be added to fortify the bread. Akunyili (2005) noted that in humans, potassium bromate causes cough and sore throat on inhalation; abdominal pain, diarrhea, nausea, vomiting, hearing loss, kidney failure, bronchial and ocular problems when ingested. Watson (2000) reported that potassium bromate possess the potential to cause cancer in experimental animals and in humans. Flour and bread treated with potassium bromate proved carcinogenic on oral administration Kurokawa et al. (2015). Chijioke (2014) demonstrated that potassium bromate induces renal tumors, mesotheliomas of the peritoneum and follicular cell tumors of the thyroid. In addition, KBrO3 also affects the kidney, heart, lungs, and other sensitive organs.
Despite warnings from food and health agencies on the health dangers in several countries including Nigeria, bromate is still in use as a dough improver Emeje et al. (2009). The world health organization (WHO) and National Agency for Food and Drug Administration Control (NAFDAC) stipulated the permissible amount of bromate in bread to be 0.02 mg/Kg Akunyili (2005). However, Ekop (2008) opined that the use of potassium bromate in bread was banned by NAFDAC in 2004. Despite this ban of the additive, the compliance level has been low. Many bread bakeries, especially those established in rural areas, still employ the additive, in large quantities, in their products in certain parts of Nigeria Naze et al. (2018). Monica (2022) reiterated that potassium bromate is a banned flour-improver and a known cancer-causing agent which can also lead to kidney failure. In this study, bread samples from five selected bakeries in Ukanafun Local Government Are of Akwa Ibom State, Nigeria were assessed for their levels of bromate.
2. METHODS
Ultra-violet spectrophotometric method, adapted from Dagari et al. (2022) was used as the analytical procedure for this work. The bread samples were those that are most commonly consumed in that locality; they were randomly selected from bakeries and safely taken to the laboratory to prevent contamination. The study centered on five popular bakeries in the community.
·
Qualitative Analysis of Potassium Bromate:
From the center of each bread loaf, 25g portion of the loaf was taken and kept for three days in the laboratory at room temperature to dry in the absence of sunlight. The dried bread crusts was pulverized with a blender. With the aid of an electronic weighing balance (model: BL-410S), 1.0g was taken and transferred to five different beakers, labeled as A, B, C, D and E. 20 ml of distilled water was added to each beaker, and allowed to stand for 30 minutes to completely dissolve the bread samples. Each sample was decanted into a 15ml centrifuge tubes and centrifuged at 3000rpm for 10minutes. Thereafter, the filtrate was separated from the residue using a Whatman no: 1 filter paper. Pipette was used to measure 5ml of the filtrate and transferred into a test tube. 5ml of freshly prepared 0.5 % potassium iodide solution in 0.1M hydrochloric acid was added to the test tube. The color change was observed for 5 minutes. The process was repeated for the other bread samples B, C, D and E, as well as for bread ‘F’ baked in the laboratory without potassium bromate.
·
Quantitative Analysis of Potassium Bromate:
The absorbance of the standards and the samples were taken at 540nm, which is the maximum absorption wavelength for potassium bromate obtained in the visible region of the spectrophotometer (model: SP721E). The absorbance were converted to concentrations with reference to a calibration curve constructed from pure potassium bromate sample solutions.
3. RESULTS AND DISCUSSION
The interaction of each bread portion with distilled water yielded a milky colored solution. However, when the bread samples interacted with the reagent, potassium bromate from the bread reacted with potassium iodide in 0.1M hydrochloric acid to yield a purple coloration which indicated the presence of bromate in the bread. The color change ranged from very light purple to deep purple reflecting a variation in the concentration of bromate among the bread samples. Bread samples A and B gave light purple color while Samples C, D and E gave deep purple color. Absorbance recorded for each bread sample along with the corresponding concentration of potassium bromate in them are displayed in figure 1. From the results bromate concentration in each bread sample varies with the purple color intensity of the filtrate. Bread samples A and B with light purple filtrate color had low absorbance of 0.516 and 0.538 respectively. However, bread samples C, D and E had deep purple filtrate color and higher absorbance values of 0.850, 0.638 and 0.796 respectively.
Figure 1
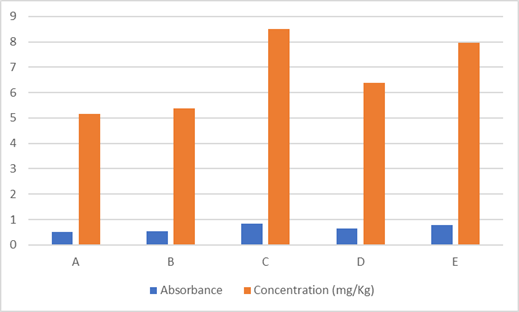
|
Figure 1 Absorbance and Concentration of Bromate in Bread Samples |
The result shows that all the bread samples assessed had bromate with concentrations above the permissible level set by NAFDAC in Nigeria. The maximum limit of potassium bromate allowed in bread around Nigeria by the NAFDAC is 0.02 mg/Kg Ekop et al. (2008). This implies that bread sold in that locality contains bromate thus consumers’ health is at risk in the community. Dagari et al. (2022) reported bromate levels of 12.16 μg/g and 0.0001μg/g as highest and the lowest level of KBrO3 found in bread samples consumed in parts of Gashua and Nguru communities of Yobe state, Nigeria. Similar Studies carried out by Naze et al. (2018) for bread samples produced in River State also gave bromate levels that are higher than safe limits; 0.025 – 0.058 mg/Kg and 0.011– 0.059 mg/Kg for Port Harcourt south and north respectively. On the other hand, Magomya et al. (2013) reported potassium bromate level of 2.46 – 13.60 mg/kg for bread samples obtained around Zaria and its environs.
4. CONCLUSION
Bread is an important carbohydrate component of diet which is baked from dough of flour and other ingredients. Potassium bromate has been used by most bakers as an ingredient of choice for bread making as dough improver.
According to Spassova et al. (2015) and Kurokawa et al. (2019) Potassium bromate ingestion can result in rapid deterioration of the kidney (nephrotoxicity), acute or chronic liver injury or impairment of liver functioning (hepatotoxicity), alteration to the normal function of the central nervous system, with potential to disrupt neurons (neurotoxicity) and uncontrolled proliferation of cells (carcinogenicity). Potassium bromate is highly irritating and injurious to the tissues especially those of the central nervous system Robert & William (2019) and as such should not be consumed by humans. In addition to several literature reports and despite its ban by NAFDAC, this study revealed that bromate is still used by bakers, especially in rural areas in Nigeria for making bread. All the bread samples analyzed contained potassium bromate above safe level and limit recommended for human consumption thus may lead to organ toxicity. Hence, there is strong need for continuous surveillance and enforcement of the ban on the use of potassium bromate in the baking industries by the National Agency for Food, Drug Administration and Control, (NAFDAC). Studies for other safe and effective dough improvers that can replace potassium bromate should be carried out.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
Akunyili, N. D. (2005). Eradication of Potassium Bromated from Nigerian Bakery Industry. NAFDAC, 5, 1-6.
Chijioke, O. (2014). Analysis of Potassium Bromate in Bread Sampled from Enugu Metropolis, 2, 34-60.
Dagari, M. S., Jafiya, L., Idris, M., & Baffa, A. A. (2022). Determination of Potassium Bromate in Bread Samples from Gashua and Nguru Communities of Yobe State, Nigeria. International Journal of Science and Technology Research Archive, 03(01), 058-065. https://doi.org/10.53771/ijstra.2022.3.1.0062
Ekop, A. S., Obot, I. B., & Ikpatt, E. N. (2008). Anti-Nutritional Factors and Potassium Bromate Content in Bread and Flour Samples in Uyo Metropolis, Nigerian. E-J Chem, 5, 736-741. https://doi.org/10.1155/2008/530596
Emeje, M. O., Ofoefule, S. I., Nnaji, A. C., Ofoefule, A. U., & Brown, S. A. (2009). Assessment of Bread Safety in Nigeria: Quantitative Determination of Potassium Bromate and Lead. African Journal of Food Science, 4, 394-397.
Emeje, O. M., Ifiora, B. I., Ezenyi, C. I., & Ofoefule, S. I. (2015). National Institute for Pharmaceutical Research and Development, Abuja, Nigeria. Department of Pharmaceutical Technology and Industrial Pharmacy, University of Nigeria, Nsukka, Enugu State, Nigeria.
Kurokawa, Y., Maekawa, A., & Takahashi, M. (2015). Toxicity and Carcinogenicity of Potassium Bromate: a New Renal Carcinogen. Environ Health Perspectives, 87, 309-315. https://doi.org/10.2307/3431039
Kurokawa, Y., Takamura, N., Matsuoka, C., Imazawa, T., Matsushima, Y., Onodera, H., & Hayashiet Y. (2019). Comparative Studies on Lipid Peroxidation in the Kidney of Rats, Mice and Hamsters and on the Effect of Cysteine, Glutathione and Diethylmaleate Treatment on Mortality and Nephrotoxicity after Administration of Potassium Bromate. International Journal of Toxicology, 6, 489-501. https://doi.org/10.3109/10915818709075694
Magomya, A. M., Yebpella, G. G., Amos, H. S., Udiba, U. U., & Latayo, M. S. (2013). Potassium Bromate and Heavy Metal Content of Selected Bread Samples Produced in Zaria, Nigeria. International Journal of Science & Technology, 2(2), 232 -237.
Naze, A. U., Epete, O. A., & Owhoeke, E. (2018). Bromate Content in Thirty Different Brands of Bread Baked In Port Harcourt Metropolis Rivers State, Nigeria. Journal of Applied Science & Environmental Management, 22(8), 1321 -1324. https://doi.org/10.4314/jasem.v22i8.29
Oloyede, O. B., & Sunmoru, T. O. (2009). The Potassium Bromate Content of Selected Bread Samples in Ilorin, Central-Nigeria and its Effect on some Enzymes of Rat Liver and Kidney. Food Chemical Toxicology, 47, 2067-2070. https://doi.org/10.1016/j.fct.2009.05.026
Robert, T., & William, J. (2019). Principles and Methods for the Assessment of Neurotoxicity Associated with Exposure to chemicals. Institute of Environmental Health, 60, 23-24.
Sai, K. A., & Takagi, T. U. (1991). Relation of 8-Hydrogen Guanosine Formation in Rat Kidney to Lipid Peroxidation, Glutathione Level and Relative Organ Weight After a Single Dose Administration of Potassium Bromate. Japanese. Journal of Cancer Research, 82(2), 165-169. https://doi.org/10.1111/j.1349-7006.1991.tb01824.x
Spassova, M. A., Miller D. J., & Nikolov A.S. (2015). Kinetic Modeling Reveals the Roles of Reactive Oxygen Species Scavenging and DNA Repair Processes in Shaping the Dose- response Curve of KBrO3-Induced DNA Damage. Oxid Med Cell Longev, 375-378. https://doi.org/10.1155/2015/764375
Vicki, S. (2019). Bromate Analysis, Food Science Technology. Bull Publications, 80, 57-80.
Watson, Y. (2000). Material Safety Data Sheet Potassium Bromate, Mallinckrodt Baker Inc. New Jersey.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2024. All Rights Reserved.