|
|
|
|
Article Type: Research Article
Article Citation: Francisco de A. Ribeiro Filho, Fernando B. Mainier, and Luciane P. Costa
Monteiro. (2020). EVALUATION OF THE USE OF GLYCERIN AND SODIUM SILICATE IN
WATER-BASED DRILLING FLUID. International Journal of Research -GRANTHAALAYAH, 8(5),
187-193. https://doi.org/10.29121/granthaalayah.v8.i5.2020.156
Received Date: 16 May 2020
Accepted Date: 31 May 2020
Keywords:
Drilling Fluid
Bi-Distilled Glycerin
Corrosion Inhibitor
Sodium Silicate
ABSTRACT
Glycerin is a by-product of the transesterification reaction of vegetable oil to produce biodiesel. Glycerin production has increased as the number of biodiesel industries has grown. With features such as water solubility, low cost, and non-toxicity, it is a good substance for water-based drilling fluid formulations with less environmental contamination. The experiments were conducted using a drilling fluid commonly used in Brazil and were compared with glycerin additions at concentrations of 5, 10, 15, and 20% by volume. Considering that as a result of the raw materials used, the biodiesel production routes produce a contaminated glycerin, it was decided to use a bi-distilled glycerin. In addition, sodium silicate, which uses industrial water or seawater, was added as a corrosion inhibitor due to its good performance and environmental non-toxicity. The sodium silicate was effective in combating corrosion without interfering with the fluid properties. The values of plastic viscosity, yield point, L3 (reading 3 rpm), and gel strength, mostly presented results equal to or better than the original formulation of the fluid used as a comparison.
1. INTRODUCTION
Drilling
fluid, or mud as it also called, is a mixture of natural and synthetic chemical
compounds used to cool and lubricate the drill bit, clean the hole bottom,
carry cuttings to the surface, control subsurface pressure, provide wellbore
stability, and minimise formation damage, etc. Drill muds are generally
classified into water-based mud (WBM) and oil-based mud (OBM). The WBM have
greater environmental acceptance but must meet certain standards in order to be
in contact with oil well drilling operations. OBM are extremely harmful to
marine life therefore it is necessary to use risers to avoid the direct contact
of the OBM with the seabed. Risers are tubular structures that connect the BOP
(Blowout Preventer) to the turntable of the platform [1], [2], [3], [4].
The muds
are usually composed of two phases: a dispersant (aqueous or organic), and a
scattered phase, the complexity of which depends on the nature of the scattered
products and its required functions.
Each
drilling fluid has a specification for the operation to be completed safely and
quickly. Therefore, it is necessary to present characteristics compatible with
the lithology and types of rocks present in the drilling area.
The main
requirements are [1], [2], [3], [4]
·
Chemical
stability;
·
Acceptance
of any chemical and physical treatment;
·
Be
easily separated from gravel on the surface;
·
Do
not cause damage to formations;
·
Be
easy to pump;
·
Have
low degree of corrosion and abrasion;
·
Facilitate
geological interpretation of cuttings and well logging;
·
Respect
environmental laws;
·
Low
cost.
Most of the
equipment used in drilling operations consisting of tanks, pumps, and pipes, is
made of carbon steel. Special steels target mainly the contamination from rocks
and water forming salinity, CO2 and H2S [1], [5], [6].
The
objective of this work is centred on the evaluation of formulated drilling
fluids containing glycerin and sodium silicate. Glycerin is a by-product of biodiesel production and its
raw material is oils from soybean, peanuts, corn, sunflower, etc.
Considering
that in Brazil the biodiesel program aims to incorporate vegetable raw material
(oilseeds) through the transesterification synthesis into conventional diesel
oil (2 to 5%), it is valid to state that there is a large availability of glycerin byproducts at a low cost
[7], [8], [9] while sodium silicate is a corrosion inhibitor
that has shown excellent performance for saline solutions.
2. MATERIALS AND METHODS
As
previously mentioned, glycerin is a by-product of
biodiesel production. The process consists essentially of oil extraction from
oilseeds (soybean, sunflower, peanut, cotton, etc.) by pressing and solvent
extraction. In order to identify the best industrial options for biodiesel
production, the composition of the triglyceride-rich produced oil, which
largely depends on the etymology of the plant species, as well as the seed oil
content and oil productivity per planted area, have to be taken into
consideration.
The
transesterification process consists of the stoichiometric reaction of three
moles of low molecular weight alcohol (methanol or ethanol) for each mole of
triglyceride in the oil, in the presence of a catalyst and at a temperature of
50 to 60°C, as shown in Figure 1. Some variables influence the
transesterification reaction, namely: reagent purity, alcohol/oil molar ratio,
reaction temperature, catalyst, and agitation [7], [8], [9].
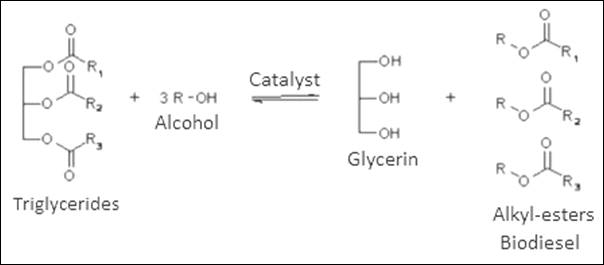
Figure 1: Transesterification
reaction
Given this
production process it can be assumed that glycerin
marketed as a by-product has a series of contaminants from the seeds used (raw
material), as well as the operating conditions of the processing (extraction
and purification).
The mud
used for this experiment is polymeric with a good performance considering the
shale lithological standard. The aim is, at least, to maintain the rheological
parameters of the original fluid, but reduce the xanthan gum concentration by
the addition of bi-distilled glycerin. The option of
using the bi-distilled glycerin rather than a
commercial product was that the commercial glycerins
that were analysed had several constituents that might hinder the process of
evaluating the mud properties.
Glycerol or
glycerin is a colourless, odourless, viscous liquid
that is sweet-tasting and non-toxic. The main physicochemical properties of the
glycerin used in the assays are presented in Table 1 [10].
Table 1: Physicochemical properties of glycerin [10].
|
Molecular formula |
C3H8O3;
CH2OH-CHOH-CH2OH |
|
Molecular Weight |
92.09 g/mol |
|
Boiling point |
290 °C |
|
Flash Point |
160 °C |
|
Melting point |
18 °C |
|
Viscosity |
1410 mPa.s at 20 ° C |
|
Density |
1.26 g/cm3
at 20 ° C |
High purity
sodium silicate hydrate (Na2SiO3.9H2O) was
used as a corrosion inhibitor. This corrosion inhibitor was chosen because it is
inorganic, non-toxic, and has had good results in the protection of carbon
steel in saline solutions [11].
Table 2, below, shows the standard drilling fluid formulation (base
fluid) used as a reference and the glycerin and
sodium silicate additions made to this standard formulation.
Table 2: Drilling Fluids Composition
|
Substances |
Function |
Base fluid |
Adding glycerin to base fluid |
|||
|
5 % |
10% |
15% |
20 % |
|||
|
Water,
v/v% |
Continuous
phase |
49 |
44 |
39 |
34 |
29 |
|
Bi-distilled
glycerin, v/v% |
Continuous
phase |
0 |
5 |
10 |
15 |
20 |
|
Saturated
sodium chloride brine, v/v% |
Continuous
phase |
40 |
40 |
40 |
40 |
40 |
|
Sodium bicarbonate,
g/L |
Hardness
removal |
0.57 |
0.57 |
0.57 |
0.57 |
0.57 |
|
Polydimethylsiloxane,
g/L |
Defoamer |
0.57 |
0.57 |
0.57 |
0.57 |
0.57 |
|
Xanthan gum,
g/L |
Viscosifier |
2.28 |
1.43 |
1.43 |
1.43 |
1.43; 1.71 |
|
Polyanionic
cellulose – low Viscosity, g/L |
Filtration
control agent |
5.70 |
5.70 |
5.70 |
5.70 |
5.70 |
|
Hydroxypropyl
starch, g/L |
Filtration
control agent |
11.41 |
11.41 |
11.41 |
11.41 |
11.41 |
|
Magnesium
oxide, g/L |
Alkaline agent |
1.42 |
1.42 |
1.42 |
1.42 |
1.42 |
|
Potassium
chloride, g/L |
Shale inhibitor |
42.80 |
42.80 |
42.80 |
42.80 |
42.80 |
|
Polyamine, g/L |
Swelling agent |
17.12 |
17.12 |
17.12 |
17.12 |
17.12 |
|
Glutaraldehyde,
g/L |
Biocide |
0.85 |
0.85 |
0.85 |
0.85 |
0.85 |
|
Calcium
carbonate, g/L |
Weighting /
bridging agent |
114.12 |
114.12 |
99.86 |
85.59 |
71.32 |
|
Polyol ester
oil, g/L |
Bit balling
preventer |
0.85 |
0.85 |
0.85 |
0.85 |
0.85 |
|
Polyethylene
glycol oleate, g/L |
Lubricant |
14.26 |
14.26 |
14.26 |
14.26 |
14.26 |
|
Sodium silicate,
g/L |
Corrosion
inhibitor |
2.28 |
2.28 |
2.28 |
2.28 |
2.28 |
A
rotational viscometer with a mud cup was used to measure the rheological
properties. The mud cup heats the fluid to the desired temperature, which is
120°F (48.88°C) according to the American Petroleum Institute (API) [12]. Measurements were taken at the following
speeds in revolutions per minute: 600, 300, 200, 100, 6 and 3. With these
measurements, it is possible to determine the plastic viscosity and the yield
point using the formulae below:
Plastic
Viscosity (PV) = L 600 – L 300.
Yield Point
(YP) = PV – L 300.
For the
base fluid, the xanthan gum concentration was 2.28
g/L. After manufacture, the fluids were placed in scroll caps and
inserted in an oven for 16 hours to simulate their ageing.
The
referral values for the main properties are summarised in Table 3.
Table 3: Properties and their target values
|
Properties |
Referral values* |
|
Plastic Viscosity |
15 – 40 cP |
|
Yield Point |
15 – 40 lb/100ft² |
|
Gel Strength (10 seconds) |
7 – 15 lb/100ft² |
|
Gel Strength (10 minutes) |
8 – 25 lb/100ft² |
|
L3, rpm |
7 – 10 |
|
Fluid Density |
9.8 – 10.2 lb/gal |
*Considering the fluid under operation
AISI 1020 carbon coupon (0.21 % of carbon) mass loss assays
were performed by immersion in the base fluids and base fluids with the
addition of 20% v/v% glycerin for 10 days.
3. RESULTS AND DISCUSSIONS
The
objective of using glycerin, besides giving value to
the product, is to keep the rheological parameters constant, and to decrease
the concentration of xanthan gum in the fluid formulation, with the aim to have
a good rheology for the drilling of an oil well.
The results of laboratory tests that can determine the conditions of the use of drilling fluid in oil well drilling operations are presented below in Figures 1, 2 and 3.
Plastic
viscosity is directly linked to the internal resistance exerted by a fluid to
flow. The solids content in a drilling fluid influences the particle friction,
a higher solid content results in a higher particle friction, which results in
a higher plastic viscosity [13].
The Yield
point (YP) indicates the ability of the fluid to carry the cuttings generated
by the drilling up to the surface. It is the minimum effort required to
initiate the fluid movement [14].
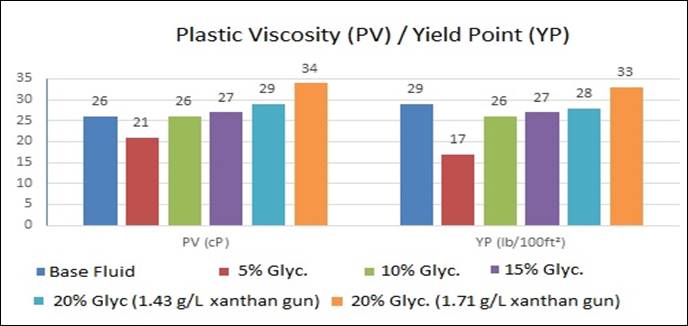
Figure 1: Plastic viscosity and Yield point
All values
obtained regarding the plastic viscosity (PV) of the fluid and the Yield Point
(YP) were within the range considered ideal for the formulation of the base
fluid. Fluids with a 10% bi-distilled glycerin
content had values similar to the base fluid. Compared to the 20% content of
bi-distilled glycerin, the influence of the xanthan
gum concentration on the properties is more evident with an increase, as seen
when comparing the concentrations of 1.43 g/L and 1.71 g/L.
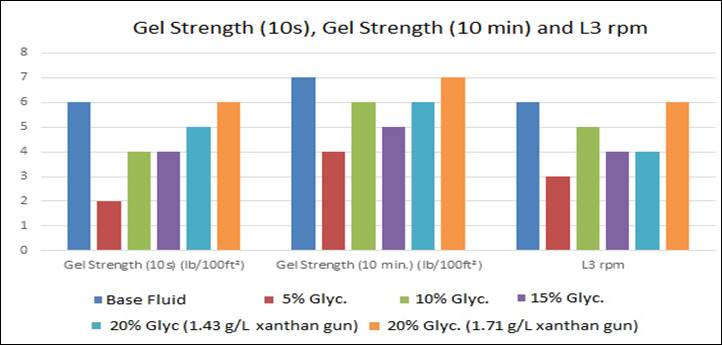
Figure 2: Gel strength and L3 rpm results
Gel
strength is a rheological parameter that indicates the degree of gelification due to the electrical interaction between the
dispersed particles. The gel strength 10s measures the resistance to flow of
the fluid, while the gel strength 10 min. measures the resistance of the fluid
to restart the flow after it has been at rest for a while. The difference
between them indicates the degree of fluid thixotropic [2], [15], [16].
The L3 rpm
reading is used to determine the 10 seconds and 10 minutes gel strengths,
however, the conditions under which they are measured are different. The
10-second gel has a lower gel level than that for 10 minutes because the
particle aggregation is lower so resulting in different values.
Only the
values obtained in the fluid with 20% bi-distilled glycerin
and 1.71 g/L xanthan gum reached the referral value.
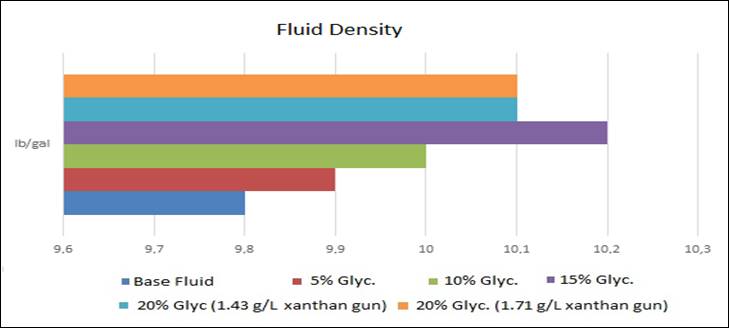
Figure 3: Fluid density results
The fluid
density values fluctuated according to the bi-distilled glycerin
content, however this is because the calcium carbonate concentrations in the
fluids were different, as can be seen in Table 1.
The desired
density value for the project can be achieved by increasing or decreasing the
amount of calcium carbonate accordingly.
The mass
losses in carbon steel coupons were negligible, evidencing that the sodium
silicate addition, at the concentration of 2.28 g/L, offers excellent
anti-corrosion protection to the carbon steel, validated by mass loss assays
performed using sodium chloride solution at 3.5 % in mass [11].
4. CONCLUSIONS
The fluid
parameters were generally within, or very close to the, stipulated ranges,
which is satisfactory for laboratory testing. In a field simulation, the
rheology would probably be slightly higher due to the solids that aggregate
during drilling.
From the
tests performed with bi-distilled glycerin on a
specific fluid formulation, it can be concluded that the presence of
bi-distilled glycerin contributes to the fluid
density, besides increasing the plastic viscosity and yield point parameters.
The fluid with a concentration of 1.71 g/L xanthan gum and 20% bi-distilled glycerin resulted in a rheology similar to that of the base
fluid (2.28 g/L of xanthan gum), but there are
possible scenarios for the results obtained with the concentration of 1.43 g/L
xanthan gum.
The
laboratory performance of the fluid can be equated with an OBM. However, the
bi-distilled glycerin fluid is far less harmful to
the environment, although the stability of the OBM is greater for drilling in
saline formations and also in the pre-salt formations.
Bi-distilled
glycerin has an advantage over crude glycerin because its properties have a standard which is
not presented by crude glycerin. In addition, the
purity percentage is close to 99%, which is higher than that of crude glycerin which has an average 30% of impurities.
SOURCES OF FUNDING
None.
CONFLICT OF INTEREST
None.
ACKNOWLEDGMENT
None.
REFERENCES
[1] Fink, J.
K. Oil field chemicals. New York: Gulf Professional Publishing, 2003.
[2]
Caenn, R. & Chillingar, G. V. Drilling fluids: State of the art. Journal
of Petroleum Science and Engineering, 14(3-4), 1996, 221-230. https://doi.org/10.1016/0920-4105(95)00051-8.
[4]
Khodja, M., Khodja-Saber, M., Canselier, J. P., Cohaut, N., &
Bergaya, F. Drilling fluid technology: performances and environmental
considerations. In: Products and services; from R&D to final
solutions, 2010, IntechOpen.
[5] Al Juhaiman, L. A.;
Mustafa, A. A.; Mekhamer, W. K. Polyvinyl pyrrolidone
as a green corrosion inhibitor for carbon steel in alkaline solutions
containing NaCl. Anti-Corrosion Methods and Materials, n. 1, v. 60, 2013,
28-36, https://doi.org/10.1108/00035591311287429
[7] Sinha, S., Agarwal, A. K., & Garg, S. Biodiesel
development from rice bran oil: Transesterification process optimization and
fuel characterization. Energy conversion and management, 49(5), 2008,
1248-1257. https://doi.org/10.1016/j.enconman.2007.08.010
[8] Meher, L. C.,
Sagar, D. V., & Naik, S. N. Technical aspects of biodiesel production by
transesterification -a review. Renewable and sustainable energy reviews, 10(3),
2006, 248-268. https://doi.org/10.1016/j.rser.2004.09.002
[9] Garcia,
E., Laca, M., Pérez, E., Garrido, A., & Peinado, J. New class of acetal
derived from glycerin as a biodiesel fuel component.
Energy & fuels, 22(6), 2008, 4274-4280. https://doi.org/10.1021/ef800477m
[10] U.S. National Library of
Medicine, National Center for Biotechnology
Information, https://pubchem.ncbi.nlm.nih.gov/compound/glycerol.
[11] Mainier, F.
B., Figueiredo, A. A., de Freitas, A. E. R., & de
Alencar Junior, A. A. M. The Use of
Sodium Silicate as a Corrosion Inhibitor in a Saline Drilling Fluid: A
Nonaggressive Option to the Environment. Journal of Environmental Protection,
7(13), 2016, 2025. http://dx.doi.org/10.4236/jep.2016.713157
[15] Jachnik, R. Drilling Fluid
Thixotropy & Relevance. Stress, 2000, 1500.
[16] Maxey, J.
Thixotropy and yield stress behavior in drilling
fluids. In AADE 2007 Drilling Fluids Conference, april
(AADE-07-NTCE-37, 2007.
|
|
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2020. All Rights Reserved.


