
A COMPREHENSIVE REVIEW ON NEUROTOXICITY OF PYRETHROIDS
Zeeshan Ahmed 1![]() , Saman Athar 2
, Saman Athar 2![]()
1 Project Assistant, Department of
Microbiology, Central Drug Research Institute, Lucknow, Uttar Pradesh, India
2 Ex-Student, Department of Zoology, Aligarh Muslim University, Aligarh, Uttar Pradesh, India
|
|
ABSTRACT |
||
|
The natural pyrethrins produced by Chrysanthemum cinerariaefolium are converted into synthetic pyrethroids. Esters of chrysanthemum acid (ethyl, 2-dimethyl-3-(1-isobutenyl) cyclopropane-1-carboxylate) and halogenated derivatives of their acids and alcohols are included in them. Pyrethroids are frequently employed in menage illnesses and companion animal ectoparasite management solutions, but their infrequent usage in domestic settings raises concerns about exposure and unfavourable effects on people and more sophisticated animals. Post convinced exploration with a wide range of pyrethroids has indicated that the choreothetosis-expectoration (CS) pattern commonly appears as chemicals with the mode T-cyano-3-phenoxybenzylalcohol, such as deltamethrin, cypermethrin, and fenvalerate. General, extensively used bracket of Pyrethroid composites are determined grounded upon the symptomology of nonentity goods noted in neurophysiological tests. Numerous lines of evidence show that all pyrethroids and DDT analogues have a single major molecular target, the voltage-sensitive sodium channel. In biophysical and biochemical examinations, the changes in sodium channel functioning are nearly connected to the impact of these substances on complete neurons. The pyrethroid sodium channel discovery point demonstrates the strict stereo particularity anticipated by in vivo nonentity neurotoxicity estimates. Composites of type I and II exhibit qualitative improvements in sodium channel tail currents, divergent effects on entire neurons and in invertebrate muscle excitability. In order to determine whether this vast and significant collection of disorders forms a single "common medium" group or several groups for the purposes of cumulative problem assessment, knowledge of the molecular processes supporting pyrethroid neurotoxicity is immediately applicable. |
|||
|
Received 10 December 2022 Accepted 10 January 2023 Published 31 January 2023 Corresponding Author Zeeshan
Ahmed, zeeshanfamily531995@gmail.com
DOI 10.29121/granthaalayah.v11.i1.2023.4924 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2023 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Pyrethroids, Neurotoxicity, Review |
|||
1. INTRODUCTION
India is a generally agricultural community. In India, new
husbandry ways similar as increased irrigation, high yielding kinds,
agrochemicals, and field mechanization have all helped to increase food product
Skolarczyk et al. (2017), Wylie et al. (2016) .
Contemporaneously, the use of pesticides on a wide scale for mosquito, disease,
and agrarian protection has expanded Mehrotra (1990),
Morgan (1992),
Skolarczyk et al. (2017). The riddle with pesticides
is that they shield police, but their wide use frequently comes with pitfalls
ranging from acute exposure to long term health consequences Mehrotra (1990), Morgan (1992), Skolarczyk et al. (2017).
Pesticides are divided into two orders: inorganic composites
and organic composites. Pesticides similar as synthetic pyrethroids and
organophosphates are generally used worldwide. The wide and expansive use of
synthetic pyrethroids and organophosphates has rebounded in resistance,
nonentity rejuvenescence, and health pitfalls Morgan (1992), Toynton et al. (2009).
Synthetic pyrethroids are created from the natural pyrethrins that Chrysanthemum
cinerariaefolium produces. Halogenated
derivatives of their acids and alcohols, as well as chrysanthemum acid esters,
are included among them Chrustek et al. (2018), Soderlund (2012), Costa (2015), Sethi et al. (n.d.). Natural
ingredients used in the Chrysanthemum cinerariaefolium extract are rapidly corrupted by light
and have been substituted with synthetic derivatives that were first thought to
be safe for humans and other sophisticated species. These contain 42 compounds
and are classified as the fourth group of germicides by the WHO (Chrustek et al., 2018). The interaction of
pyrethroids with sodium channels and the generation of sustained depolarization
in neurons serve as their mechanisms of action. Cárcamo et al. (2017), Chrustek et al. (2018), Soderlund (2012), Hussain (n.d.), Sethi et al. (n.d.), Wylie et al. (2016).
Pyrethroids have been used to discourage pests and minimize
crop losses for over a century Spurlock and
Lee (1991). It has been set
up that numerous known pyrethroids are dangerous to humans, mammals, marine
creatures, and other healthy organisms Frank et al. (2018). Likewise, their
prolonged presence in the ecosystem can affect in pollution, similar as
impurity of groundwater, dislocation of agrarian product, and so on. Despite
all sweats to minimize the negative impact of germicides on the terrain,
there's still a pressing need to probe safer germicides Ishaaya (2003).
Pyrethroids mostly enter the body through skin contact,
although they can also do so by inhalation or ingesting food or drink. Hughes et al. (2008), Chrustek et al. (2018), Hughes and Edwards (2010), Hughes and Edwards (2016), Orsborne et al. (2016), Ranjkesh et al. (2013), Singleton et al. (2014). Professional
job, water, nutrition, and household are the main exposure types Chrustek et al. (2018). It was established that the
metabolites of pyrethroids were found in the urine following ingestion of
semolina (pasta), rice, bread, morning cereals, and fruits from various
locations. Chrustek et al. (2018), Glorennec et al. (2017), Del Prado-Lu (2015).
2. THE STATUS OF PYRETHROID INSECTICIDES CURRENTLY
Synthetic pyrethroid insecticides have been widely employed
for more than three decades to reduce nonentity pests and disease vectors.
Their use has increased to account for 18% of the market's total cash value by
2002 Soderlund (2012), Pickett (2004). Pyrethroids are
effective against malaria and other microbiological diseases in addition to
their use in pest management. The common use of pyrethroids in household
germicides and companion animal ectoparasite control treatments, along with
their restricted usage in the home environment, increases the risk of exposure
and negative effects in the general populace. Soderlund (2012), Naeher et al. (2010), Ostrea et al. (2009), Power and Sudakin (2007). The Food
Quality Protection Act (FQPA) of 1996 authorised nonsupervisory evaluation of
the enrollments of pyrethroid germicide products in
the United States. Since these categories of fungicides are thought to share a
"common medium of toxicity," this legislation mandates that the
United States Environmental Protection Agency take over the accretive danger
evaluations for them.
When assessing whether this broad and significant class of
germicides represents a single "common medium" group or numerous
groups for the purposes of accretive threat assessment, knowledge of the
molecular processes underlying pyrethroid neurotoxicity is immediately
applicable. The default presumption of the reduced perceptivity of foetuses and
newborns to the harmful effects of fungicides is a
different nonsupervisory element added to the FQPA. By addressing this
dereliction presupposition at the pharmacodynamic position, knowledge of the discriminational perceptivity of fetal,
neonatal, and adult targets for the neurotoxic conduct of pyrethroids gives the
power to improve the accuracy of threat assessments Soderlund (2012).
Marketable specifics, such as household fungicides, pet
sprays, and cleansers, include nonentity control pyrethroids. Some pyrethroids
are also used as nonentity repellents that may be applied to clothing and for
direct lice treatments. The conflation analogues and derivations represent a
diversified group over other strong germicides from the original pyrethrins. Although they're grounded on chemical changes
that make them more dangerous and less environmentally degradable The Pesticide Manual World
Compendium. (1997).
3. STRUCTURE AND
INSECTICIDAL PROPERTIES OF PYRETROIDS
3.1. STRUCTURE AND CLASSES OF PYRETROIDS
Pyrethroids can be classified into two types on the basis of their mode of action Table 1. Type I are based upon electrophysiological criteria. These compounds cause restlessness, in coordination and hypersensitivity followed by prostration and paralysis Wylie et al. (2016). Type II are classified on the basis of symptoms observed in pests, these compounds produce convulsive effects within minutes of dosing Ray (1991).
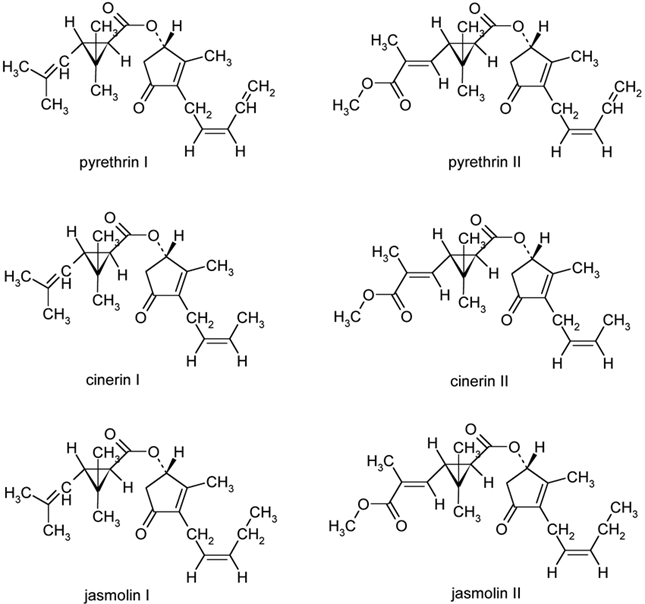
Pyrethrins, cinerins,
and jasmolins are all contained in a common extract
of natural pyrethrum from Chrysanthemum cinerariaefolium. Pyrethrin I is the most effective
natural insecticide while pyrethrin II delivers more of the desired quick
knock-down action against flying insects. The structure of pyrethrin I is an
excellent starting point for addressing the molecular features required for
insecticidal action, as well as how these features can be changed to maximise
efficacy, reduce mammalian toxicity, and enhance stability. This will show how
the guiding principles that contributed to the current range of synthetic
pyrethroids were created. This will show how the guiding principles that
contributed to the current range of synthetic pyrethroids were created Elliott (1971), Elliott (1976), Sawicki, and Thain (1962).
Table 1
|
Table 1 Classification of Synthetic Pyrethroids |
|||
|
Pyrethrins |
Type I Pyrethroids |
Type II Pyrethroids |
Reference |
|
Constituents of natural pyrethrum extract |
Derivatives of pyrethrins that do not include a cyano group and may elicit tumors |
Derivatives of pyrethrins that include a cyano group and may elicit sinuous writhing and salivation |
|
|
Pyrethrin I |
Allethrin |
Cyfluthrin |
(Sonia Sethi, n.d.) |
|
Pyrethrin II |
Bifenthrin |
Deltamethrin |
|
|
Cinerin I |
Permethrin |
Cypermethrin |
|
|
Cinerin II |
Pheothrin |
Fenvalerate |
|
|
Jasmolin I |
Resemethrin |
Fenpropathrin |
|
|
Jasmolin II |
Tefluthrin |
Flucythrinate |
|
|
|
Tetramethrin |
Flumethrin |
|
Figure 1
|
Figure 1 Structures of Natural Pyrethrins |
Figure2
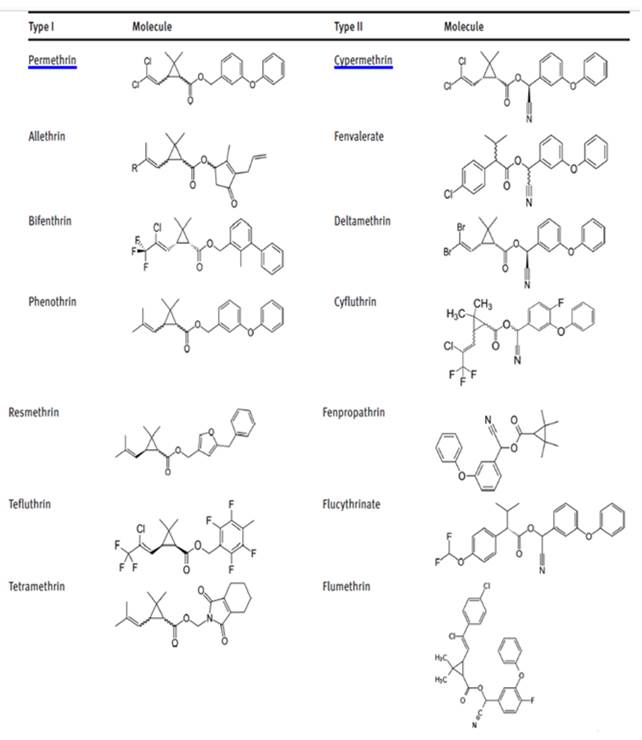
|
Figure 2 Pyrethroids Type I and Type II |
Due to the many chiral carbons in cyclopropyl, the majority
of pyrethroids have several stereoisomers. In addition, some pyrethroids have
olefinic structures that are susceptible to cis or trans isomers. And because
the activities or toxicity of different stereoisomers to different organisms
are quite unequal, enrichment of active isomers can improve insecticidal activity
while reducing toxicity to non-target organisms to some extent Gerlach (2012), Nillos et al. (2008), Gan and Schlenk (2008).
In the case of cyhalothrin, two chiral carbon atoms on the
cyclopropane ring produce four optical isomers and two geometric isomers,
yielding a total of eight isomers. There is also a chiral carbon atom in the
cyanohydrin structure, which produces two optical isomers, R- and S-. As a
result, there are sixteen optical isomers of cyhalothrin (eight pairs of
optical enantiomers) Lutnicka and K. A. (2009).
3.2. SYNTHESIS OF
PYRETHROIDS
To produce a concentrate comprising the six pyrethrin types—pyrethrin I, pyrethrin II, cinerin I, cinerin II, jasmolin I, and jasmolin II—organic detergents are used to uproot the active components of pyrethroids. The three rethrolones are pyrethrolone, jasmolone, and cinerolone. Pyrethrins that contain chrysanthemic acid are classified as type I, and those that contain pyrethric acid are classified as type II. Processing the flowers to grow the pyrethrin is a time- consuming process that varies by area.
The six esters that make up natural pyrethrins are composed of a monoterpenoid acid that has been partially conjugated to an oxylipin alcohol of the rethrolone class. Early feeding exploration suggests that the three rethrolones (pyrethrolone, jasmolone, and cinerolone) are derived from the octadecanoid pathway, while the two monoterpenoids (chrysanthemic acid and pyrethric acid) are derived from the plastidial 1-deoxy-D- xylulose-5-phosphate (DXP) terpenoid pathway Barthel (1961), Goffinet and Locatelli (1969), Martel and Huynh (1967), Schechter et al. (1949).
Figure 3
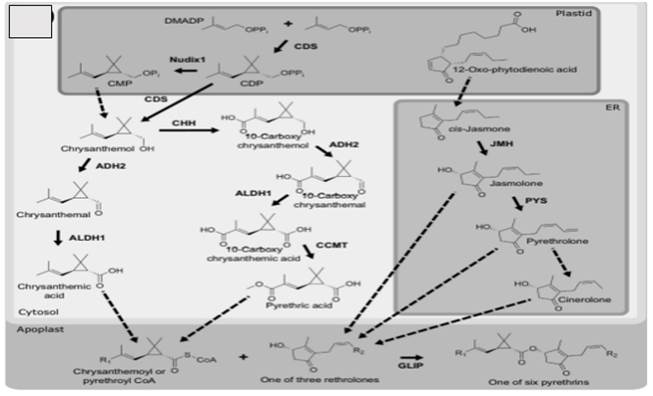
|
Figure 3 Synthesis of Natural Pyrethrins |
The configurations of natural esters have been used to produce a variety of synthetic pyrethroid. Allethrin, constructed by Schechter, Green, and La Forge in 1949, was the first important synthetic emulsion that's still used moment. Natural esters pentadienyl side chains were condensed and simplified to make allethrin Barthel (1961), Toynton et al. (2009). Allethrolone, the alcoholic portion, is now commercially resolved, and the (+)- form, esterified with synthetic (+)- trans- chrysanthemic acid (+), yields S- bioallethrin, which is stereochemically original to pyrethrin I. Allethrin, bioallethrin, and S- bioallethrin are produced in lesser amounts than any other synthetic pyrethroid. Allethrin is used as a relief for natural pyrethrum when stocks are limited. Allethrin is frequently used in mosquito coils because of its advanced volatility and thermal adaptability than natural esters. 9 S- Bioallethrin is a quick knockdown agent for certain insects, but it lacks the wide range of action of natural pyrethrins, including bioallethrin and allethrin Elliott (1971), Rauch et al. (1972). The coming synthetic pyrethroid to be commercially developed was Neopynamin (tetramethrin), which was published in 1964. Although neopynamin knocks insects down snappily when mixed into aerosols and analogous phrasings, the alcoholic portion isn't structurally related to other synthetic pyrethroids, and it isn't inescapably a strong payoff agent Elliott (1976), Fales et al. (1972), Kato et al. (1964). Presently, large-scale civilization of Dalmatian pyrethrum is demanded for artificial product of pyrethrin germicides. Before being collected, gutted, and ground up, pyrethrins build up in mature flower heads to a mass of one to two dry pounds Chang et al. (2014), Sethi et al. (n.d.). The powdered material can then be sold straight or combined with organic detergents to make insecticidal detergents and sprays Kaneko (2010). In addition to dry maquillages and liquid sprays used for small-scale ground-position care or large-scale upright operation compass of nonentity pests, pyrethrins are added to various accessories such as poultices and mosquito coils for specific nonentity safety. Wider spectrum germicides that work against a variety of non-entity pests include Coleoptera, Dyptera and Hemiptera (Homoptera and Heteroptera), Hymenoptera, Lepidoptera and Orthoptera, and Thysanoptera Bradberry et al. (2005), Kaneko (2010), Kaneko (2011), Toynton et al. (2009). They are scattered on food products before harvesting to control pests and also are used as germicides and protectives for homes. They are used on beast houses, in granges, in green houses and in veterinary drug considerably Table 2. Pyrethroid effectiveness and selectivity are influenced by factors such as shape, significant structural features such as the ester and non-ester, specific chiral stereochemistry across the cyclopropane ring, physical parcels (for example, flying nonentity pests make good composites), and chemical parcels (e.g., more polar emulsion for knockdown). In order to boost the efficacy of germicides, the pyrethroids have been synthesised with compounds comparable to piperonyl butoxide, piperonyl sulfoxide, and sesamex. The pyrethroids used in commercial formulations contain a significant amount of additional, mostly toxic, inactive components Costa (2015).
Table 2
|
Table 2 Applications of Pyrethroids |
||||
|
Pyrethroid |
Insects |
Crops |
Other locations and applications |
Reference |
|
Allethrin |
Flies, mosquitoes,ants |
NIA |
Tropical use in pet sprays and shampoos, residential, public
health, animal housing |
(Sonia Sethi, n.d.) |
|
Bifenthrin |
Beetles, weevil, houseflies, mosquitoes, lice, bedbugs, aphids, moths, cockroaches, locust |
Alfalfa, haybeans, cantaloupes, cereals, cotton field and grassseed, hops, melons,
watermelons. |
NIA |
|
|
Bioresmethrin |
Houseflies, mosquitoes, cockroaches |
NIA |
Household, public health, animal houses |
|
|
Cyfluthrin |
Aphids, cabbage stem flea beetle, cockroaches, houseflies,
mosquitoes, rape winter stem weevil |
Alfalfa, cereals, cotton, citrus, deciduous fruit, growing nuts, maize, oilseed, pears, potatoes, rice, sugarbeet, sugarcane,
tobacco, vegetables. |
Green houses |
|
|
Cyhalothrin |
Bedbugs, beetles, houseflies, lice, mosquitoes, moths, weevils |
NIA |
Public health, animal houses, inert surfaces |
|
|
Cypermethrin |
Cockroaches, flies, mosquitoes, moths |
Cotton, lettuce, onions, pears, peaches, pecans, sugarbeets |
Residential and commercial buildings, animal’s houses |
|
|
Deltamethrin |
Aphids, beetles, bollworm, bud-worm, caterpillars, cicadas, totrix moths, weevils, whitefly, winter moths |
Alfalfa, beet, cereals, coffee, cotton, figs, fruits, hops,
maize, oilseed rape, olives, oil pahns, potatoes, rice,
soybeans, sunflowers, tea, tobacco, vegetables. |
Forests, households, animal houses, stored products |
|
|
Esfenvalerate |
Beetles, moths |
Cabbage, cotton, fruit trees, grains, groundnuts, maize, potatoes,
sorghum, soybeans, sugarcane, sunflowers, tomatoes, vegetables, wheat. |
Ornamentals, non crop land. |
|
|
Fenvalerate |
Beetles, cockroaches, flies, locusts, mosquitoes, moths |
Alfalfa hay, apples, beet, cereals, cotton, cucurbita,
fruit, greenbeans, groundnuts, hops, maize, nuts, oilseed
rape, olives, potatoes, sorghum, soybeans, squash, sugarcane, sunflower, vegetables,
vines, tobacco. |
Ornamentals, forestry, non-crop land. |
|
3.3 MECHANISM OF
ACTION IN INSECTS
Pesticides that have been introduced to cropland can be picked
up by plants Burridge and H. K. (1997), eaten by
animals, insects, or microorganisms in the soil, or travel downward in the soil
Lidova et al. (2016) and either
sticks to it or dissolves in water, or vaporises Lutnicka and K. A. (2009) and enters the
atmosphere, or breaks down into less toxic compounds through microbial and
chemical pathways or is leached out Orsborne et al. (2016), Wang et al. (2017) .The stability
and solubility properties of pesticides applied to soil play a big role in
their destiny.
Pyrethrum compounds are inactivated and decomposed by
exposure to light and air and are also broken down in water to nontoxic
materials. Mild acids and alkalis degrade pyrethrins
quickly as well Burridge and H. K. (1997), Lidova et al. (2016). Voltage-gated sodium channels are the
primary target of pyrethroids neurotoxic effects on insects. Voltage-gated
calcium and voltage-gated chloride channels are additional targets Burridge and H. K. (1997), Lidova et al. (2016), Lutnicka and K. A. (2009). Pyrethroids
affect sodium ion channels in insect nervous systems, causing them to open
faster, stimulating nerve cells and causing paralysis. Calcium signals control
a number of neuronal growth pathways, while chloride channels regulate cell
length, resting potential, and transepithelial transport Orsborne et al. (2016), Wang et al. (2017).
1) Type I
pyrethroids: Permethrin is a member of the first category of
pyrethroids. This substance exists as a liquid as well as yellow-brown and
brown crystals and is soluble in organic solvents Toynton et al. (2009). Permethrin
comes in two optical stereoisomers; cis and trans. Studies show that cis-permethrin is more neurotoxic than trans-permethrin Nasuti et al. (2013). Additionally,
permethrin nanometrics with hydrodispersive
characteristics are employed to stop the chemical from binding. Therefore, it
may be promptly eliminated from the body because colloidal water dispersion
prevents it from accumulating and reduces its period of retention in the body. Davies et al. (2007).
Skin, the
digestive system, and the respiratory system are the three main routes through
which the permethrin enters the body Toynton et al. (2009), Wylie et al. (2016). By accelerating
impulse conduction, permethrin damages insect nerves, causing paralysis and
eventual death. Voltage-gated sodium channels, such as Nav1.6, Nav1.3, and
Nav1.8 in mammals and VGSCs in insects, are affected by the substance by
causing them to open too early and delaying their inactivation Soderlund (2012), Costa (2015), Sethi et al. (n.d.). This pyrethroid operates on calcium
channels by promoting calcium ion return transport, according to research to
date. Soderlund (2012), Costa (2015), Power and Sudakin (2007).
2) Type II
pyrethroids: Deltamethrin is a member of the first class of pyrethroids
(Figure 1B). This medication is soluble in alcohol and acetone but lipophilic
and insoluble in water. Colorless, white, and/or
medium beige crystals have been discovered Nillos et al. (2008), O'Reilly et al. (2006). It is employed
in farming. All of these pests are successfully
controlled, including aphids, whiteflies, lice, tse-tse
flies, fleas, ticks, spiders, ants, bees, bedbugs, and cockroaches. Mosquito
nets are frequently treated with deltamethrin because it also protects against
malaria vectors including Aedes aegyptii and
Anopheles gambiae Table 2 Gebreslassie et al. (2012).
It is believed
that the origin of deltamethrin's neurotoxic action is the prolonged activation
of voltage-gated sodium channels, which results in neuronal membrane
depolarization, repetitive discharges, synaptic abnormalities, and hyperexcitatory poisoning symptoms in insects. Soderlund (2012), Costa (2015). Deltamethrin
also influences the function of calcium and chloride channels in neurons.
Pyrethroids are
2250 times more toxic to insects than higher mammals. Insects' smaller anatomy,
more fragile sodium channels, and lower body temperatures account for this. Sethi et al. (n.d.)s. Pyrethroids have been shown to
have a detrimental effect on the ion channels in neuronal membranes as well as
the mitochondrial membranes of aquatic species including fish (carp and rainbow
trout) and shellfish (crayfish, lobster).Burridge and H. K. (1997), Cárcamo et al. (2017), Lidova et al. (2016), Lutnicka and K. A. (2009), Toynton et al. (2009), Wang et al. (2017).
4. NEUROTOXICITY OF PYRETHROIDS
Neurotoxicity may be described as any unfavourable effects
generated by chemical, biological or physical agents on the central or
peripheral nervous system. Neurotoxicity has been demonstrated to have been
associated with several substances, including metals (e.g. plum), industrial
chemicals (e.g. acrylamide), solvents, natural poisons (e.g. domoic acid),
pharmaceutical medicines (e.g. doxorubicin), misuse medicaments (e.g. ectasy), and pesticides World Health Organization (WHO).
(2016). Because of some
fundamental properties including aerobic metabolism reliance, existence of
axonal transport and the neurotransmission process, the nervous system is
particularly susceptible to injury Singh et al. (2012).
Moreover, the nervous system develops, which is believed to
be more susceptible to neurotoxic chemicals in terms of replication, migration,
differentiation, neuronal myelination, and synapse. Increased is that the
blood-brain barrier is not fully established. In fact, some of the known
neurotoxicants are predominantly developmental and quantitatively and
qualitatively distinct symptoms of neurotoxicity in development and adult years
(e.g., in case of lead or ethanol).
Neurotoxicants can be divided in four groups from a general
mechanical perspective. These groupings are neuronopathic, axon-targeted and axonopathic, myelinopathic and
neurotransmission affective. A series of chemicals can lead to toxicity that
leads to loss of neurons by necrosis or by apoptosis (neuronopathy). This loss
of neurons is irreversible and may lead to a loss of certain functions through
a global encephalopathy or, if neuron subpopulations alone are affected Singh et al. (2012).
4.1.
EFFECT ON NERVES
A crucial framework for examining molecular mechanisms of
action is provided by the detrimental effects of pyrethroids on normal nerve
function. Extracellular electrodes were used in early electrophysiological
studies of pyrethrum's effects on nerve function to measure the compound nerve
effect potential in pesticide- and pencil-prepared ventric
nerve cords. The induction of recurrent releases, measured either as an
increase in spontaneous activity or a range of potential for action generated
by the one electric stimulation, which is followed by an electrical block, is
one of the key characteristics of pyrethral and
pyrethroid intoxication on the whole nerve level. These conclusions also
include the fundamental reason for observing the effects of allethrin on nerve
action potentials, especially modifies the transitory sodium conductance
through the voltage-sensitive sodium channel. These findings were validated in
a range of axonal preparations by subsequent research using numerous Type I
Pyrethroids Narahashi (1962a), Narahashi (1962b), Ruigt (1985).
Pyrethroids have well-characterized effects on nerve axons and also affect other neuronal components. Both invertebrate
and vertebrate sensory structures are extremely sensitive to pyrethroids and
typically react differently than axonal preparations. The housefly larva
sensor nerves, the cercal nerves of cockroach and the locust crural nerve sensory fibres create extremely extended
spontaneously or ambiguously triggered high-frequency impulse trains. Two
reaction patterns were found in comparison with the activity of a number of pyrethroid structures. Short sense explosions
were related with Type I compounds in cockroach preparation (e.g. alethrin, tetramethrin), whereas locust chemicals
categorised as Type II generated extended sensory explosions Clements and May (1977), Gammon et al. (1981), Osborne and Hart (1979).
Pyrethroids also influence synaptic transmission via the
presynaptic nerve endpoint. Pyrethroid depolarization is carried out in motor
terminals linked to insect larval wall body muscles, resulting in release of
neurotransmitters. These effects are assessed as the first rise in the
frequency of small postsynaptic excitatory potential in muscles, followed by
neuromuscular blocking. Pyrethroids effects on the sodium channel led to
nervous-terminal depolarization, as demonstrated by the fact that tetrodotoxin
(TTX) prevented the sodium channel's pyrethroid-dependent function. Salgado et al. (1983).
In addition, the activities of the DDT must include any
consideration of pyrethroid activity on nerves. Early physiological trials with
DDT and pyrethroids have shown that these structurally varied chemicals
have had remarkably comparable effects on invertebrate axons and frog
peripheral nerves. The later development of a number of hybrid DDT-pyrethroid
insecticides that have structural components that may be exploited for
insecticidal activities in both DDT and pyrethroid demonstrate another another similar method of action for these two pesticide
classes. Holan et al. (1978), Narahashi (1969), Van Den Bercken et al. (1979).
5. MECHANISM NEUROTOXICITY
OF PYRETHROIDS
5.1.
EFFECT ON INSECTS VOLTAGE GATED SODIUM CHANNELS
A pore generating sub-unit (sub-unit) plus an auxiliary
sub-unit make up VGSCs Dong (2007). The component
is divided into four domains (I-IV), each of which has six trans-membrane
segments. The protein's amino and carboxy termini are found within the cell. The
fifth and sixth transmembrane segments (S5 and S6), as well as the loop between
them, which provides selectivity for Na+, together make up the
channel pore Dong (2007). As a voltage sensor, the positively
charged amino acids in S4 cause a conformational shift that causes the channel
to open when the membrane depolarizes. Domains III and IV are connected by a
cytoplasmic connection that acts as an inactivity gate. The two separate
"gates" that regulate the four states in which VGSCs can reside are
known as the activation gate and the inactivation gate. The channel is closed,
and the door is open at the resting membrane potential. The channel opens when
the membrane becomes depolarized, enabling Na+ to enter the cell.
The VGSC α subunit is encoded by a single gene in
insects. This gene is known as para in Drosophila melanogaster. The
mature transcripts from this gene's messenger RNA are alternatively divided
into different combinations of exons. The transcripts go through an RNA editing
process, which normally changes the base of the encoded amino acid in certain
nucleotides. Alternate splitting and RNA editing are used to create VGSCs with
unique gating properties. In Drosophila
melanogaster, the auxiliary component TipE
enhances the expression of the Para cell surface, heightens the Na+ current
peak, and changes the kinetics of channel inactivation.
Users of a fire-polized glass
micropipette can see the currents created by separately opening and shutting
sodium channels in tiny pieces of a cell membrane by using patch clamping
technology. Hamill et al. (1981). [1R,trans]-Tetramethrin created a population of changed sodium
channels under patch cluster circumstances, in which sodium conductance and
opening kinetics were unaffected but channel-open distribution durations were
noticeably lengthened. Zamponi et al. (1997). In a
preliminary communication, extension of one-channel currents by fenvalerate was also documented Holloway et al. (1984). The results of
patch clamp studies contradict the pyrethroid's predicted effects on sodium
channels and instead show that the pyrethroid only modifies sodium channel
inactivation kinetics, with no appreciable impact on other characteristics.
Table 3
|
Table 3 List of some of the species with kdr mutations |
|||
|
Mutation |
Location in VGSC |
Species |
References |
|
Leucine to Phenylalanine |
DIIS6 |
Musca domestica, Blattella germanica, Plutella xylostella, Myzus persicae, Anopheles gambiae, Culex pipiens, Culex quinquefasciatus, Haematobia irritans, Leptinotarsa decemlineata, Ctenocephalides felis Frankliniella occidentalis, Cydia pomonella. |
Dong (2007) |
|
Leucine to Serine |
DIIS6 |
Culex pipiens, Anopheles gambiae |
|
|
Leucine to Histidine |
DIIS6 |
Heliothis virescens |
|
|
Methionine to Threonine |
DIIS4-S5 linker |
Musca domestica, Haematobia irritans, Heliothis virescens |
|
|
Aspartate to Glycine |
Amino terminus |
Blattella germanica |
|
|
Glutamate to Lysine |
DI and DII linker |
Blattella germanica |
|
|
Cysteine to Arginine |
DI and DII linker |
Blattella germanica |
|
|
Proline to Leucine |
Carboxy terminus |
Blattella germanica |
|
|
Valine to Methionine |
DIS6 |
Heliothis virescens |
|
|
Methionine to Isoleucine |
DIIS1-S2 linker |
Pediculus capitis |
|
|
Leucine to Phenylalanine |
DIIS5 |
Pediculus capitis |
|
|
Threonine to Isoleucine |
DIIS5 |
Plutella xylostella, Pediculus capitis |
|
|
Threonine to Cytosine |
DIIS5 |
Frankliniella occidentalis |
|
|
Threonine to Valine |
DIIS5 |
Ctenocephalides felis |
|
|
Phenylalanine to Isoleucine |
DIIIS6 |
Boophilus microplus |
|
|
Leucine to Proline |
DIII-DIV linker |
Varroa destructor |
|
There are genetic indications, in addition to
electrophysiological data, showing pyrethroids target the VGSC. In the 1950s
houseflies were initially documented in pyrethroid, or knockdown-resistant
(KDR) Busvine (1951), Davies et al. (1958). The flying
family's kdr and superkdr
traits have shown that the VGSC VSsc1 housefly was directly responsible for their
resistance to pyrethroids Williamson et al. (1993). Similar genetic
mapping studies linked kdr and super-kdr traits in those species' VGSC genes in mosquitoes,
German cockroaches, and tobacco budworms Dong and Scott (1994), Severson et al. (1997), Taylor et al. (1993). Two-point mutations were
detected with Domain II by comparative sequencing analysis of Vssc1 kdr and super-kdr housefly with
the wild-style gene Williamson et al. (1996). The first mutation, a change in
phenylalanine in transmembrane segment 6, was seen in the two kdr and six super-kdr housefly
strains. Only super-Kdr strains were found to have
the second mutation, which changes methionine to threonine in the intracellular
loop between transmembrane segments 4 and 5. In the sequence of VGSC genes from
several species with kdr resistance, further changes Table 3 have been found Dong (1997), Dong (2007), Ingles et al. (1996), Miyazaki et al. (1996), Park et al. (1997), Soderlund et al. (2008).
5.2. EFFECT ON GABA
RECEPTOR- IONOPHORE COMPLEX
In recent years, intensive research has been done to
ascertain whether or not the GABA receptor chlorides
ionophore complex of inhibitory synapses is a primary or secondary target for
pyrethroids of type II. Although functional testing has indicated that the
interaction of type II pyrethroids with the GABA mammalian receptor complex has
been established in binding assays, this connection may not have much of a toxicologenic effect Gammon et al. (1982). Assessment of
the effects of pyrethroid interactions with the chloride channel's TBPS bonding
site is possible using chloride ion flow studies to test GABA receptor
connections in mammalian brain preparations to their chloride canals Bloomquist et al. (1986), Harris and Allan (1985).
The most thorough analysis was used to compare the activities
of the pyrethroid effects of deltamethrin, its benign enantiomer, and its
insecticide noncyano counterpart, NRDC 157 Bloomquist and Soderlund
(1985). GABA induced
chloride absorption via deltamethrin inhibition into the mouse brain vesicles
was inadequate and could not exceed 60% inhibition at 30/~ M.
The Type I ester NRDC 157 is predicted to be an inhibitor of
chloride absorption by TBPS binding studies. Despite the likelihood that
pyrethroid interactions with the TBPS site may alter the GABA receptoryophore's ability to function, our results
demonstrate that deltamethrin is 1000 times less efficient as a sodium channel
activator than as an inhibitor of GABA-dependent chloride absorption. The
incomplete stereospeutic properties of deltamethrin
and its enantiomer inhibit chloride uptake are not compatible with the absolute
stereospeutic character of both neurotoxicity and
sodium channels, as determined by the results of intact nerves and the impact
of these compounds Ghiasuddin and Soderlund
(1985).
Pyrethroids only have a very strong effect on this target,
according to functional tests with GABA-invertebrate species. Although the
concentrations of deltamethrin affecting the GABA musculoskeletal receivers
were several orders greater than those causing a deep disruption of the
crayfish nerves through an effect on sodium channels that depended on voltage,
the actions of GABA at the crayfish neuromuscular junction were antagonised by
deltamethrin and other Type II pyrethroids. Purves et al. (2001).
5.3. PERIPHERAL TYPE
BENZODIAZEPINE RECEPTORS
Pyrethroids lower the BBC Ro5-ability 4864's ability to bind
to the "benzodiazepine receptor peripheral-type" site but have no
effect on the binding of benzodiazepine radioligands to the benzodiazepine
recognition site associated with the GABA receptor complex. Gammon et al. (1981), Lawrence et al. (1985). The capacity of
type I and type II pyrethroids to behave as proconvulsants
has recently been linked to their ability to interact with this site by
lowering the threshold for the onset of pentylenetetrazole-induced
seizures in rats. Devaud and Murray (1987), Devaud et al. (1986).
Stereospecificity for neurotoxic isomers is suitable in the proconvulsing
and receptor-binding effects both. It is important to acquire maximised in vivo
provoking effects at dosages much below the levels necessary for
pyrethroid-dependent acute poisoning Devaud et al. (1986). Additionally,
Ro5-4864 was shown to delay the onset of detamethrine
and permithrin in the cockroaches and to stop the
GABA ligand flunitrazepam from binding to preparations of insect nerve and muscle
receptors in mammals. The relevance of those findings as proof of pyrethroid
interactions with either the GABA receptor complex or an insect-type peripheral
benzodiazepine receptor is uncertain because benzodiazepines are difficult to
correlate with mammalian functions in insects Abalis et al. (1983), Lummis and Sattelle (1986).
5.4. NICOTINIC
ACETYLCHOLINE RECEPTORS
Pyrethroid interactions with the nicotinic acetylcholine
receptor were examined in the process of attaching the [3H] perhydrohistrionico
toxin (H2-HTX) to a locus connected to the acetylcholine gated ion channel. Pyrethrins, alethrin, resmethrin, and Tetramethrin were the most potent and rapid
inhibitors of H2-HTX binding, but permethrin and other cyan-replaced esters
were less potent and shown slow association. kinetics Abbassy et al. (1982). These
pyrethroids also decreased Carbachol triggered 45Ca2+ intake, an acetilcholine analogue supposed to mimic flow via the
relatively unspecified cholinergic receptor cation canal. However, after tests
demonstrated that pyrethroids had no effects on 22Na+ absorption,
which indicated that there was really a seeming impact on calcium absorption on
calcium binding Eldefrawi et al. (1985). These findings
suggest that the H2-HTX binding domain of the nicotinic acetylcholine receiver
interacts with pyrethroids without affecting ion transport.
5.5. THE
REGULATION OF CALCIUM AND ATP HYDROLYZING ENZYMES
The many Ca2+ ATPases are a group of ATPases
involved in the action of pyrethroids and are thought to have a role in the
stringent homeostatic regulation of calcium levels intracelled.
Early research demonstrating the effects of DDT and pyrethroids on Ca2+
dependent ATPases as well as the causes for the control of calcium in pesticide
action have all been covered elsewhere Beeman (1982). Pyrethroids
inhibit two Ca2+ATPase activities in squid and cockroach nerve
preparations: a Na+-Ca2+-ATPase that was supposed to be
an ATP-module sodio-calcium tractor and was more sensitive to alleethrin; and a Ca2+Mg2+ ATPase
that was supposed to represent energy-dependent extrusion of calcium and was
more sensitive to inhibition by type II pyrethroid Clark and Matsumura (1982).
Comparing the effectiveness of several pyrethroids as inhibitors
in this test revealed that the Type II compounds were more potent than the Type
I ones. The studies also showed that pyrethroids decreased the activity of
adenylate cyclase when it was induced by calmodulin, indicating that
interactions with calmodulin rather than ATPase or adenylate cyclase have acted
as a mediator for all activities in this system. Additionally, DDT and
pyrethroids lessen the activation of phosphodiesterase by calmodulin Rashatwar and Matsumura (1985).
6. CONCLUSION
For at least two centuries, the insecticide capabilities of
the pyrethrins, the natural insecticides present in
pyrethrum flowers and other extracts, were identified. A variety of synthetic
analogues were produced and tested between 1940 and 1970 by pyrethrins
(named pyrethroids). Some of the compounds have showed an outstanding
insecticide activity, including allethrin, tetramethrin, and resmethrin and have been produced as commercial home,
storage, and veterinary insecticides. However, they all had one thing in
common: natural pyrethroid esters are unstable in the environment, which
prevents them from being widely employed in agricultural production. The global
agricultural chemicals industry reaffirmed its interest in this kind of
material, prompting Permethrin, the first photostable pyrethroid, to conduct
extensive research and development. A number of
previous pyrethroids and other kinds of insecticides have been superseded by
the various photostable pyrethroids that were produced as a consequence of
these efforts, and they have proven to be incredibly efficient farm insecticides.
As a result, pyrethroids have a comprehensive identification of one of the four
or five main classes of synthetic insecticides.
Various lines of evidence suggest the major location of
pyrethroid activity in the neurological system. In insects, pyrethroids quickly
cause indications of poisoning, indicating action on the neurological system
(lack of coordinated movement, convulsive activities phases and final
paralytic). Post induced research with a wide range of pyrethroids has
indicated that the choreothetosis-salivation (CS)
syndrome often occurs as substances like deltamethrin, cypermethrin, and fenvalerate, which have the mode T-cyano-3-phenoxybenzylalcohol.
General, widely used classification of Pyrethroid compounds (a structurally
various group producing a syndrome) and compounds of the Type II compounds
(predominately Cyan-3-phenoxybenzyl esters, producing a CS syndrome), are
determined based upon the symptomology of insect effects noted in
neurophysiological tests.
However, all these activities may not be implicated in
disrupting nerve activity, pyrethroid insects interact with a range of
neurochemical procedures. The voltage-sensitive sodium channel is the main
molecular target for all pyrethroids and DDT analogues in both insects,
according to a number of lines of evidence.
Biophysical and biochemical investigations have shown that the effects of these
medicines on intact neurons are closely tied to changes in sodium channel
activity. The pyrethroid sodium channel detection site demonstrates the strict
stereo specificity expected by in vivo insect neurotoxicity estimates. Type I
and type II chemicals differ in their qualitative effects on intact neurons,
sodium channel tail currents, and the excitability of the muscles in the
vertebrate skeleton. Additionally, sodium channel kinetics has considerable
effect on the Type I/Type H categorization method. The diverse poisoning
symptoms seen in insects appear to be sufficiently explained by the multiple
sensory and motor nerve pathways of these qualitatively variable effects on the
sodium channel.
An in vitro test, the impacts of pyrethroids on additional
neurochemical targets seems not to be significant in vivo for these chemicals neurotoxin effects. Although pyrethroids Type II
interactions with the GABA receptor complex's ionophore chloride component can
inhibit GABA-dependent chloride flow, the quantities required to affect this
system are several magnitude levels higher than those that would disrupt sodium
channel function. At addition, pyrethroid activities in the GABA receptor do
not show the strict stereo specificity indicated by the acute neurotoxicity
measurements.
The link between pyrethroids and the nicotinic acetylcholine receptor does not seem to affect their functioning, unlike the activity of pyrethroids in a GABA receptor complex. This result is in accordance with the failure of physiological studies to identify the effects of pyrethroids on post-synaptic receptors, and it shows that these interactions with the nicotinic acetylcholine receptor are not harmful. Finally, there are murky connections between the neurotoxicity of DDT and pyrethroids and their inhibitory effects on ATPases and calmodulin. DDT and pyrethroid effects on mitochondrial Na+, K+, and Mg2+ ATPase have been identified, although these effects do not fully explain their effects on the nerves as a whole. Although regulating intraneuronal calcium concentration is more directly related to nerve function, pyrethroids may not directly account for those drugs' effects on neuronal excitability by altering calcium homeostasis.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
Abalis, I. M., Eldefrawi, M. E., and Eldefrawi, A. T. (1983). Biochemical Identification of Putative GABA/Benzodiazepine Receptors In House Flythorax Muscles. Pesticide Biochemistry and Physiology, 20(1), 39-48. https://doi.org/10.1016/0048-3575(83)90119-0.
Abbassy, M. A., Eldefrawi, M. E., and Eldefrawi, A. T. (1982).
Allethrin Interactions With The Nicotinic Acetylcholine Receptor Channel. Life
Sciences, 31(15), 1547-1552. https://doi.org/10.1016/0024-3205(82)90045-5.
Barthel, W. F. (1961). Synthetic Pyrethroids. Advan. Pest Control Res, 4, 33.
Beeman, R. W. (1982). Recent Advances In
Mode of Action of Insecticides. Annual Review of Entomology, 27, 253-281. https://doi.org/10.1146/annurev.en.27.010182.001345.
Bloomquist, J. R., Adams, P. M., and Soderlund, D. M. (1986). Inhibition of 3,-Aminobutyriacc Id-Stimulated Chloride Flux In Mousbe Rain Vesicle By Polychlorocycloalkane And Pyrethroid Insecticides. Neurotoxicology, 7(3), 11-20.
Bloomquist, J. R., and Soderlund, D. M. (1985). Neurotoxic Insecticides Inhibit GABA-Dependent Chloride Uptake by Mouse Brain Vesicles. Biochemical and Biophysical Research Communications, 133(1), 37-43. https://doi.org/10.1016/0006-291x(85)91838-8.
Bradberry, S. M., Cage, S. A., Proudfoot, A. T., and V. J. A. (2005). Poisoning Due To Pyrethroids. Toxicology Research, 24, 93-106. https://doi.org/10.2165/00139709-200524020-00003.
Burridge, L. E., and H. K. (1997). Lethality of Pyrethrins to Larvae and Postlarvae of The American Lobster (Homarus Americanus). Ecotoxicology and Environment Safety, 38, 150-154. https://doi.org/10.1006/eesa.1997.1571
Busvine, J. R. (1951). Mechanism of resistance to insecticide in houseflies. Nature, 168(4266), 193-195. https://doi.org/10.1038/168193a0.
Chang, F., Dutta, S., Becnel, J. J., Estep, A.
S., and Mascal, M. (2014). Synthesis Of The Insecticide Prothrin and Its
Analogues from Biomass-Derived 5-(Chloromethyl) Furfural. Journal of
Agricultural and Food Chemistry, 62(2), 476-480. https://doi.org/10.1021/jf4045843.
Chrustek, A., Hołyńska-Iwan, I.,
Dziembowska, I., Bogusiewicz, J., Wróblewski, M., Cwynar, A., and
Olszewska-Słonina, D. (2018). Current Research on The Safety of
Pyrethroids Used as Insecticides. Medicina, 54(4). https://doi.org/10.3390/medicina54040061.
Clark, J. M., and Matsumura, F. (1982). Two Different Types of Inhibitory Effects of Pyrethroids on Nerve Ca- and Ca + Mg-Atpase Activity In The Squid, Loligo Pealei. Pesticide Biochemistry and Physiology, 18(2), 180-190. https://doi.org/10.1016/0048-3575(82)90104-3.
Clark, J. M., and Matsumura, F. (1987). The Actin of Two Classes of Pyrethroids on The Inhibition of Brain Na-Ca and Ca + Mg ATP Hydrolyzing Activities of The American Cockroach. Comp. Biochemistry and Physiology, 86, 13545. https://doi.org/10.1016/0742-8413(87)90156-3.
Clements, A. N., and May, T. E. (1977). The Actions of Pyrethroids Upon The Peripheral Nervous System and Associated Organs In The Locust. Pesticide Science, 8(6), 661-680. https://doi.org/10.1002/ps.2780080611.
Costa, L. G. (2015). The Neurotoxicity of Organochlorine and Pyrethroid Pesticides. Handbook of Clinical Neurology, 131, 135-148. https://doi.org/10.1016/B978-0-444-62627-1.00009-3.
Cárcamo, J. G., Aguilar, M. N., Carreño, C. F., Vera, T., Arias-Darraz, L., Figueroa, J. E., Romero, A. P., Alvarez, M., and Yañez, A. J. (2017). Consecutive Emamectin Benzoate and Deltamethrin Treatments Affect The Expressions and Activities of Detoxification Enzymes in the Rainbow Trout (Oncorhynchus Mykiss). Comparative Biochemistry and Physiology. Toxicology And Pharmacology, 191, 129-137. https://doi.org/10.1016/j.cbpc.2016.10.004.
Davies,
M., Keiding, J., and Von Hosten, C. G. (1958). Resistance To Pyrethrins and
To Pyrethrinspiperonyl Butoxide in a Wild Strain of Musca Domestica L. In
Sweden. Nature, 182(4652), 1816-1817. https://doi.org/10.1038/1821816a0.
Davies, T. G. E., Field, L. M., Usherwood, P.
N. R., and Williamson, M. S. (2007). DDT, Pyrethrins, Pyrethroids and
Insect Sodium Channels. IUBMB Life, 59(3), 151-162. https://doi.org/10.1080/15216540701352042.
Del Prado-Lu, J. L. (2015). Insecticide residues in soil,
water, and eggplant fruits and farmers’ health effects due to exposure to
pesticides. Environmental Health and Preventive Medicine, 20(1), 53-62. https://doi.org/10.1007/s12199-014-0425-3.
Devaud, L. L., Szot, P., And Murray, T. F.
(1986). PK 11195 Antagonism of Pyrethroid-Induced Proconvulsant
Activity. European Journal of Pharmacology, 121(2), 269-273. https://doi.org/10.1016/0014-2999(86)90499-1.
Devaud, L., and Murray, T. F. (1987). Interactions of Pyrethroid Insecticides With The Peripheral-Type Bcnzodiazepine Receptor. Social Neuroscience [Abstr.], 13, 1230.
Dong, K. (1997). A Single Amino Acid Change In The Para Sodium Channel Protein Is Associated With Knockdown-Resistance (Kdr) To Pyrethroid Insecticides In German Cockroach. Insect Biochemistry and Molecular Biology, 27(2), 93-100. https://doi.org/10.1016/s0965-1748(96)00082-3.
Dong, K. (2007). Insect Sodium Channels
and Insecticide Resistance. Invertebrate Neuroscience, 7(1), 17-30. https://doi.org/10.1007/s10158-006-0036-9.
Dong, K., and Scott, J. G. (1994). Linkage of Kdr-Type
Resistance and the Para-Homologous Sodium Channel Gene In German Cockroaches (Blattella
Germanica). Insect Biochemistry And Molecular Biology, 24(7), 647-654. https://doi.org/10.1016/0965-1748(94)90051-5.
Eldefrawi, M. E., Sherby, S. M., Abalis, I. M., and Eldefrawi, A. T. (1985). Interactions of Pyrethroid and Cyclodiene Insecticides With Nicotinic Acetycholine and GABA Receptors. Neurotoxicology, 6(2), 47-62.
Elliott, M. (1971). The Relationship Between The Structure and the Activity of Pyrethroids. Bulletin of the World Health Organization, 44(1-3), 315-324.
Elliott, M. (1976). Properties and Applications of Pyrethroids. Environmental Health Perspectives, 14, 3-13. https://doi.org/10.2307/3428357.
Fales, J. H. et al. (1972). Relative Effectiveness of Pyrethroid Insecticides. Soap, Cosmetics, Chemical Specialties, 48, 60.
Frank, D. F., Miller, G. W., Harvey, D. J., Brander, S. M., Geist, J., Connon, R. E., and Lein, P. J. (2018). Bifenthrin Causes Transcriptomic Alterations In Mtor and Ryanodine Receptor-Dependent Signaling and Delayed Hyperactivity In Developing Zebrafish (Danio Rerio). Aquatic Toxicology, 200, 50-61. https://doi.org/10.1016/j.aquatox.2018.04.003.
Gammon, D. W., Brown, M. A., And Casida, J. E. (1981). Two Classes of Pyrethroid Action In the Cockroach.Biochern. Pesticide Biochemistry and Physiology, 15(2), 181-191. https://doi.org/10.1016/0048-3575(81)90084-5.
Gammon, D. W., Lawrence, L. J., and Casida, J.
E. (1982). Pyrethroid Toxicology: Protective Effects of Diazepam and
Phenobarbital In The Mouse and the Cockroach. Toxicology and Applied
Pharmacology, 66(2), 290-296. https://doi.org/10.1016/0041-008x(82)90294-0.
Gebreslassie, B. H., Yao, Y., and You, F. (2012). Design Under Uncertainty of Hydrocarbon Biorefinery Supply Chains: Multiobjective Stochastic Programming Models, Decomposition Algorithm, and A Comparison Between Cva R and Downside Risk. Aiche Journal, 58(7), 2155-2179. https://doi.org/10.1002/aic.13844.
Gerlach, R. W. (2012). Chiral Chemistry and Toxicity Assessments for Pyrethroid Pesticides. Journal of the American Chemical Society. https://doi.org/10.1021/bk-2012-1099.ch002.
Ghiasuddin, S. M., and Soderlund, D. M. (1985). Pyrethroid
Insecticides, 24, 200-206. https://doi.org/10.1016/0048-3575(85)90129-4.
Glorennec, P., Serrano, T., Fravallo, M., Warembourg, C., Monfort, C., Cordier, S., Viel, J. F., Le Gléau, F., Le Bot, B., and Chevrier, C. (2017). Determinants of Children's Exposure To Pyrethroid Insecticides In Western France. Environment International, 104, 76-82. https://doi.org/10.1016/j.envint.2017.04.007.
Goffinet, B., and Locatelli, A. (1969). Separation of Dtrans-Chrysanthemic Acid From Its Optical and Geometrical Isomers. Chem [Abstr.]. Fr Pat. 1,536,458, 71, 90923w.
Hamill, O. P., Marty, A., Neher, E., Sakmann, B., and Sigworth, F. J. (1981). Improved Patch-Clamp Techniques for High Resolution Current Recording From Ceils and Cell-Free Membrane Patches. Pflügers Archiv - European Journal of Physiology, 391(2), 85-100. https://doi.org/10.1007/BF00656997.
Harris, R. A., and Allan, A. M. (1985).
Functional Coupling of 3,-Aminobutyric Acid Receptors to Chloride Channels In
Brain Membranes. Science, 228(4703), 1108-1110. https://doi.org/10.1126/science.2581319.
Holan, G., O'Keefe, D. F., Virgona, C., and
Walser, R. (1978). Structural and Biological Link Between Pyrethroids
and DDT In New Insecticides. Nature, 272(5655), 734-736. https://doi.org/10.1038/272734a0.
Holloway, S. F., Salgado, V. L., Wu, C. H., and Narahashi, T. (1984). Maintained Opening of Single Na Channels By Fenvalerate. Social Neuroscience [Abstr.], 10, 864.
Hughes, E. A., Flores, A. P., Ramos, L. M., Zalts, A., Richard Glass, C., and Montserrat, J. M. (2008). Potential Dermal Exposure To Deltamethrin and Risk Assessment For Manual Sprayers: Influence of Crop Type. Science of the Total Environment, 391(1), 34-40. https://doi.org/10.1016/j.scitotenv.2007.09.034.
Hughes, M. F., and Edwards, B. C. (2010). In Vitro Dermal Absorption of Pyrethroid Pesticides In Human and Rat Skin. Toxicology and Applied Pharmacology, 246(1-2), 29-37. https://doi.org/10.1016/j.taap.2010.04.003.
Hughes, M. F., and Edwards, B. C. (2016). In Vivo Dermal Absorption of Pyrethroid Pesticides In the Rat. Journal of Toxicology and Environmental Health. Part A, 79(2), 83-91. https://doi.org/10.1080/15287394.2015.1109571.
Hussain, M. (n.d.). Environmental Degradation : Realities and Remedies/Mumtaz Hussain (1st Ed).
Ingles, P. J., Adams, P. M., Knipple, D. C., and Soderlund, D. M. (1996). Characterization of Voltage-Sensitive Sodium Channel Gene Coding Sequences from Insecticide-Susceptible And Knockdown Resistant House Fly Strains. Insect Biochemistry and Molecular Biology, 26(4), 319-326. https://doi.org/10.1016/0965-1748(95)00093-3.
Ishaaya,
I. (2003). Introduction: Biorational Insecticides-Mechanism and
Application. Archives of Insect Biochemistry And Physiology, 54(4), 144. https://doi.org/10.1002/arch.10111.
Pickett, J. A., (2004). New Opportunities In Neuroscience, But A Great Danger That Some May Be Lost. In D. J. Beadle, I. R. Mellor and P. N. R. Usherwood (Eds.), Neurotox 2003: Neurotoxicological Targets From Functional Genomics And Proteomics . Society Of Chemical Industry. 1-10.
Kaneko, H. (2010). Pyrethroid Chemistry and Metabolism. In. In R. Krieger (Ed.), Hayyes' Handbook of Pesticide Toxicology (3rd Ed). https://doi.org/10.1016/B978-0-12-374367-1.00076-8.
Kaneko,
H. (2011). Pyrethroids: Mammalian Metabolism and Toxicity. Journal of Agricultural
and Food Chemistry, 59(7), 2786-2791. https://doi.org/10.1021/jf102567z.
Kato, T., Ueda, K., and Fujimoto, K. (1964). New Insecticidally Active Chrysanthemates. Agricultural and Biological Chemistry, 28(12), 914-915. https://doi.org/10.1080/00021369.1964.10858319.
Lawrence, L. J., Gee, K. W., and Yamamura, H. I. (1985). Interactions of Pyrethroid Insecticides With Chloride Ionophore-Associated Binding Sites. Neurotoxicology, 6(2), 87-98.
Lidova, J., Stara, A., Kouba, A., and V. J. (2016). The Effects of Cypermethrin on Oxidative Stress and Antioxidant Biomarkers In Marbled Crayfish (Procambarus Fallax F. Virginalis). Neuro Endocrinology Letters, 37(Suppl1), 53-59.
Lummis, S. C. R., and Sattelle, D. B. (1986). Binding sites
for [3H]GABA[3, H]flunitrazepam and [35S]TBPS in insect CNS. Neurochemistry International,
9(2), 287-293.
https://doi.org/10.1016/0197-0186(86)90065-3.
Lutnicka, H., and K. A. (2009). Pyrethroids as A Predisposing Factor In Fish Diseases. Ochr. Środ. Zasobów Natl, 41, 285-292.
Martel, J., and Huynh, C. (1967). Synthese de l'acide chrysanthemique lI. Bull. Soc. Chem., 985.
Mehrotra, K. N. (1990). Pyrethroids Resistant Insect Pest Management. Pesticide Research Journal, 2(1), 44-52.
Miyazaki, M., Ohyama, K., Dunlap, D. Y., and Matsumura, F. (1996). Cloning and Sequencing of The Paratype Sodium Channel Gene from Susceptible and Kdr-Resistant German Cockroaches (Blattella Germanica) and House Fly (Musca Domestica). Molecular and General Genetics, 252(1-2), 61-68. https://doi.org/10.1007/s004389670007.
Morgan, M. D. R. (1992). The BMA Guide To Pesticides, Chemicals and Health. Title. Published on Behalf of The British Medical Association By Edward Arnold.
Morgan, M. K. (2012). Children's Exposures to Pyrethroid Insecticides at Home: A Review of Data Collected In Published Exposure Measurement Studies Conducted In The United States. International Journal of Environmental Research and Public Health, 9(8), 2964e2985. https://doi.org/10.3390/ijerph9082964.
Naeher, L. P., Tulve, N. S., Egeghy, P. P., Barr, D. B., Adetona, O., Fortmann, R. C., Needham, L. L., Bozeman, E., Hilliard, A., and Sheldon, L. S. (2010). Organophosphorus and Pyrethroid Insecticide Urinary Metabolite Concentrations In Young Children Living In A Southeastern United States City. Science of the Total Environment, 408(5), 1145-1153. https://doi.org/10.1016/j.scitotenv.2009.10.022.
Narahashi, T. (1962a). Effect of The Insecticide Allethrin on Membrane Potentials of Cockroach Giant Axons. Journal of Cellular and Comparative Physiology, 59, 61-65. https://doi.org/10.1002/jcp.1030590108.
Narahashi,
T. (1962b). Nature of The Negative After-Potential Increased By The
Insecticide Allethrin in Cockroach Giant Axons. Journal of Cellular and Comparative
Physiology, 59, 67-76.
https://doi.org/10.1002/jcp.1030590109.
Narahashi, T. (1969). Mode of Action of Ddta Nd Allethrin
On Nerve: Cellular And Molecular Mechanisms. Residue Reviews, 25, 275-288. https://doi.org/10.1007/978-1-4615-8443-8_21.
Nasuti, C., Carloni, M., Fedeli, D.,
Gabbianelli, R., Di Stefano, A., Serafina, C. L., Silva, I., Domingues, V., and
Ciccocioppo, R. (2013). Effects of Early Life Permethrin Exposure on
Spatial Working Memory and on Monoamine Levels In Different Brain Areas of
Pre-Senescent Rats. Toxicology, 303, 162-168. https://doi.org/10.1016/j.tox.2012.09.016.
Nillos, M. G., Gan, J., and Schlenk, D. (2008).
Chemical Analysis and Enantioselective Toxicity of Pyrethroids. Journal of The
American Chemical Society, 991, 400-414. https://doi.org/10.1021/bk-2008-0991.ch018.
O'Reilly,
A. O., Khambay, B. P., Williamson, M. S., Field, L. M., Wallace, B. A., and Davies,
T. G. (2006). Modelling Insecticide-Binding Sites In The Voltage-Gated
Sodium Channel. Biochemical Journal, 396(2), 255-263. https://doi.org/10.1042/BJ20051925.
Orsborne, J., Deraedt Banks, S., Hendy, A., Gezan, S. A., Kaur, H.,
Wilder-Smith, A., Lindsay, S. W., and Logan, J. G. (2016). Personal Protection
of Permethrin-Treated Clothing Against Aedes Aegypti, The Vector of Dengue And Zika
Virus, In The Laboratory. PLOS ONE, 11(5), E0152805. https://doi.org/10.1371/journal.pone.0152805.
Osborne, M. P., and Hart, R. J. (1979). Neurophysiological Studies of The Effects of Permethrin Upon Pyrethroid Resistant (Kdr) and Susceptible Strains of Dipteran Larvae. Pesticide Science, 10(5), 407-413. https://doi.org/10.1002/ps.2780100507.
Ostrea, E. M., Jr., Bielawski, D. M., Posecion, N. C., Jr., Corrion, M., Villanueva-Uy, E., Bernardo, R. C., Jin, Y., Janisse, J. J., and Ager, J. W. (2009). Combined Analysis of Prenatal (Maternal Hair and Blood) and Neonatal (Infant Hair, Cord Blood And Meconium) Matrices To Detect Fetal Exposure of Environmental Pesticides. Environmental Research, 109(1), 116-122. https://doi.org/10.1016/j.envres.2008.09.004.
Park,
Y., Taylor, M. F., and Feyereisen, R. (1997). A Valine 421 to Methionine
Mutation In IS6 of the Hscp Voltage-Gated Sodium Channel Associated With
Pyrethroid Resistance In Heliothis Virescens F. Biochemical and Biophysical
Research Communications, 239(3), 688-691. https://doi.org/10.1006/bbrc.1997.7511.
Power, L. E., and Sudakin, D. L. (2007). Pyrethrin and Pyrethroid Exposures In The United States: A Longitudinal Analysis of Incidents Reported to Poison Centers. Journal of Medical Toxicology, 3(3), 94-99. https://doi.org/10.1007/BF03160917.
Purves, D., Augustine, G. J., Fitzpatrick, D., Hall, W. C., LaMantia, A.-S., Mooney, R. D., Platt, M. L., and L. E. W. (2001). Neuroscience (2nd editio). Oxford University Press.
Ranjkesh, M. R., Naghili, B., Goldust, M., and Rezaee, E. (2013). The Efficacy of Permethrin 5% Vs. Oral Ivermectin For the Treatment of Scabies. Annals of Parasitology, 59(4), 189-194.
Rashatwar, S. S., and Matsumura, F. (1985).
Interaction of DDT and Pyrethroids With Calmodulin and Its Significance In The
Expression of Enzyme Activities Of Phosphodiesterase. Biochemical Pharmacology,
34(10), 1689-1694.
https://doi.org/10.1016/0006-2952(85)90635-5.
Rauch, F., Lhoste, J., and Birg, M. L. (1972). Proprietes Insecticides Du D-Trans Chrysanthemate De D-Allethrolone. Mededel. Fak. Landbouw. Wetenschap. Gent, 37, 755.
Ray, D. E. (1991). Pesticides derived from plants and other organisms. Academic Press. (International Association of Bridge, Structural, Ornamental and Reinforcing Iron Workers J. H. Journal of Engineering Research L. (Eds.). (Ed.)).
Ruigt, G. S. F. (1985). Pyrethroids. In Comprehensive Insect Physiology G. A. Kerkut and L. I. Gilbert (Eds.), Biochemistry and pharmacology, 12, 183-262.
Salgado, V. L., Irving, S. N., and Miller, T. A. (1983). The Importance of Nerve Terminal Depolarization In Pyrethroid Poisoning of Insects. Pesticide Biochemistry and Physiology, 20(2), 169-182. https://doi.org/10.1016/0048-3575(83)90021-4.
Sawicki, R. M., and Thain, E. M. (1962). Insecticidal Activity of Pyrethrum Extract And Its Four Insecticidal Constituents Against House Flies. IV. Knock-Down Activities of The Four Constituents. Journal of The Science of Food And Agriculture, 13(5), 292-297. https://doi.org/10.1002/jsfa.2740130504.
Schechter,
M. S., Green, N., and La Forge, F. B. (1949). Constituents of Pyrethrum
Flowers. Journal of The American Chemical Society, XXIV Cinerolone and The
Synthesis of Related Cyclopentenolones, 71, 3165. https://doi.org/10.1021/ja01177a065.
Sethi, S., Mathur, N., and P. B. (n.d.). Synthetic pyrethroids: A review. International Journal of Scientific and Engineering Research, January, 20(1).
Severson, D. W., Anthony, N. M., Andreev, O., and Ffrench-Constant, R. H. (1997). Molecular Mapping of Insecticide Resistance Genes In The Yellow Fever Mosquito (Aedes Aegypti). Journal of Heredity, 88(6), 520-524. https://doi.org/10.1093/oxfordjournals.jhered.a023148.
Singh, A. K., Tiwari, M. N., Prakash, O. S.M., and Singh, M. P. (2012). A Current Review of Cypermethrin-Induced Neurotoxicity and Nigrostriatal Dopaminergic Neurodegeneration. Current Neuropharmacology, 10(1), 64-71. https://doi.org/10.2174/157015912799362779.
Singleton, S. T., Lein, P. J., Farahat, F. M., Farahat, T., Bonner, M. R., Knaak, J. B., and Olson, J. R. (2014). Characterization of Α-Cypermethrin Exposure In Egyptian Agricultural Workers. International Journal of Hygiene And Environmental Health, 217(4-5), 538-545. https://doi.org/10.1016/j.ijheh.2013.10.003.
Skolarczyk, J., Pekar, J., and Nieradko-Iwanicka, B.. (2017). Immune Disorders Induced by Exposure to Pyrethroid Insecticides. Postepy Hig Med Dosw (Online), 71(0)(I), 446-453. https://doi.org/10.5604/01.3001.0010.3827
Soderlund,
D. M. (2008). Pyrethroids, Knockdown Resistance and Sodium Channels. Pest
Management Science, 64(6), 610-616. https://doi.org/10.1002/ps.1574.
Soderlund, D. M. (2012). Molecular Mechanisms of
Pyrethroid Insecticide Neurotoxicity: Recent Advances. Archives of Toxicology,
86(2), 165-181.
https://doi.org/10.1007/s00204-011-0726-x.
Spurlock, F., and Lee, M. (2008). Synthetic Pyrethroid Use
Patterns, Properties, and Environmental Effects. ACS (Am. Chem. Soc.) Symp. [Ser.]. https://doi.org/10.1021/bk-2008-0991.ch001.
Taylor, M. F., Heckel, D. G., Brown, T. M.,
Kreitman, M. E., and Black, B. (1993). Linkage of Pyrethroid Insecticide
Resistance To a Sodium Channel Locus In The Tobacco Budworm. Insect Biochemistry
and Molecular Biology, 23(7), 763-775. https://doi.org/10.1016/0965-1748(93)90064-y.
The Pesticide Manual World Compendium. (1997). C. D. S. Tomlin (Ed.).
Toynton, K., Luukinen, B., Buhl, K., and S. D. (2009). Permethrin technical fact sheet. National Pesticide Information Center.
Wang, Y., Lv, L., Yu, Y., Yang, G., Xu, Z., Wang,
Q., and Cai, L. (2017). Single and Joint Toxic Effects of Five Selected
Pesticides on The Early Life Stages of Zebrafish (Denio Renio). Chemosphere,
170, 61-67. https://doi.org/10.1016/j.chemosphere.2016.12.025.
Williamson,
M. S., Denholm, I., Bell, C. A., and Devonshire, A. L. (1993). Knockdown
Resistance (Kdr) To DDT and Pyrethroid Insecticides Maps To A Sodium Channel
Gene Locus In The Housefly (Musca Domestica). Molecular and General Genetics,
240(1), 17-22.
https://doi.org/10.1007/BF00276878.
Williamson,
M. S., Martinez-Torres, D., Hick, C. A., And Devonshire, A. L. (1996). Identification
of Mutations In The Housefly Para-Type Sodium Channel Gene Associated With
Knockdown Resistance (Kdr) To Pyrethroid Insecticides. Molecular and General
Genetics, 252(1-2), 51-60. https://doi.org/10.1007/BF02173204.
World Health Organization (WHO). (2016). Pesticide Evaluation Scheme, Vector Ecology and Management. World Health Organization.
Wylie, B. J., Hauptman, M., Woolf, A. D., and Goldman, R. H. (2016). Insect Repellants During Pregnancy In The Era of The Zika Virus. Obstetrics and Gynecology, 128(5), 1111-1115. https://doi.org/10.1097/AOG.0000000000001685.
Zamponi, G. W., Bourinet, E., Nelson, D., Nargeot, J., and Snutch, T. P. (1997). Crosstalk Between G Proteins and Protein Kinase C Mediated By The Calcium Channel Α(1) Subunit. Nature, 385(6615), 442-446. https://doi.org/10.1038/385442a0.
Van Den Bercken, J., Kroese, A. B. A., and
Akkermans, L. M. A. (1979). Effects of Insecticides on The Sensory
Nervous System. In Neurotoxicology of Insecticides and Phermones, 210, 183-210. https://doi.org/10.1007/978-1-4684-0970-3_10.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2023. All Rights Reserved.