
BIOACCUMULATION FACTOR (BAF) IN FISH CAUGHT IN A RIVER IMPACTED BY EFFLUENTS FROM AN ALUMINA PLANT IN THE EASTERN BRAZILIAN AMAZON
Cléber Silva e Silva 1,2,3 ![]()
![]() ,
Simone de Fátima Pinheiro Pereira 2,3,4
,
Simone de Fátima Pinheiro Pereira 2,3,4![]()
![]() , Pedro Moreira de Sousa Junior 2,5
, Pedro Moreira de Sousa Junior 2,5![]()
![]() ,
Alan Marcel Fernandes de Souza 6
,
Alan Marcel Fernandes de Souza 6![]()
![]() , Daniel Pinheiro Nogueira 2
, Daniel Pinheiro Nogueira 2![]()
![]() ,
Davis Castro dos Santos 2,3,7
,
Davis Castro dos Santos 2,3,7![]()
![]() ,
Ronaldo Magno Rocha 2,4,8
,
Ronaldo Magno Rocha 2,4,8![]()
![]()
1 Federal Institute of Education, Science, and Technology of Pará. Almirante Barroso Avenue, 1155 - Marco, Belém, PA, Brazil
2 Environmental and Analytical Chemistry Laboratory, Federal University of Pará. Augusto Correa Street, S/N - Guamá, Belém, PA, Brazil
3 Postgraduate Program in the National Network for the Teaching of Environmental Sciences, Federal University of Pará. Augusto Correa Street, S/N - Guamá, Belém, PA, Brazil
4 Chemistry Postgraduate Program, Federal University of Pará. Augusto Correa Street, S/N - Guamá, Belém, PA, Brazil
5 Federal Rural University of the Amazon. Barão de Capanema Avenue S/N - Caixa D'Água, Capanema, PA, Brazil
6 University of the Amazon. Alcindo Cacela Avenue, 287 - Umarizal, Belém, PA, Brazil
7 Federal University of Pará - Altamira Campus, Coronel José Porfírio Street, 2515 - São Sebastiao, Altamira, PA, Brazil
8 Central Laboratory of the Pará Health Department. Augusto Montenegro Avenue, 524 - Parque Guajará, Belém, PA, Brazil
|
|
ABSTRACT |
||
|
The rivers of
the Amazon are important water resources for the planet however they are
gradually suffering from anthropic impacts, especially those arising from
mining and industrial activity. In this study, the bioaccumulation factor of
toxic elements in tissues of fish species collected in the Murucupi River, a
local impacted by effluents from an alumina factory located in BARC arena, in
the Brazilian Amazon, was evaluated. Twenty samples were collected from three
species of fish Cichla spp, Eigenmannia sp., and Angelfish. The element Al,
Cr, Cu, Fe, Mn, Ni, Pb, and Zn were analyzed in fish tissue and gills using
inductively coupled plasma optical emission spectrometry. Regarding the
concentration of the elements evaluated in the tissue, only Pb was not in
compliance with the legislation. The BAF for the fish tissue samples
indicated Cu bioaccumulation for the species Cichla spp (1130 L.kg-1) around
seven times higher than the established limit, Eigenmannia sp. (2885 L.kg-1)
fourteen times larger, and Angelfish (1640 L.kg-1) eight times larger. Ni
also showed bioaccumulation for the specie Cichla spp (150 L.kg-1) and
Eigenmannia sp. (145 L.kg-1) around one and a half times higher than
recommended for both species. Zn showed bioaccumulation for the species Cichla
spp (4212 L.kg-1), Eigenmannia sp. (3538 L.kg-1) around four times higher for
both species, and Angelfish (7942 L.kg-1) around eight times higher. These
elements with BAF above the recommended can present risks to the biota and
consumers. |
|||
|
Received 18 April 2022 Accepted 17 May 2022 Published 15 June 2022 Corresponding Author Alan
Marcel Fernandes de Souza, alanmarcel2@gmail.com DOI 10.29121/granthaalayah.v10.i5.2022.4632 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2022 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Environmental Impact, Contamination,
Toxic Elements, Rivers |
|||
1. INTRODUCTION
Significant amounts of toxic elements in wastewater are discharged into rivers around the world as result of the large population growth accompanied by the evolution of industrial and agricultural production Omar et al. (2015), Yi and Zhang (2012) These metals can accumulate in water and sediments and bio magnify in aquatic food chains, resulting in sublethal effects or death in local fish populations Kumari (2018), Stankovic et al. (2014)
In recent years, much attention has been paid to concentrations of toxic elements in fish and other foods with greater interest in those that pose a risk to human health. Studies report that long-term toxic elements cause neurological disorders, cancer, degenerative diseases, and other toxic effects on the body Khaneghaha and Sant'ana (2020)
The species composition and temporal distribution of aquatic organisms are altered by the actions of impacts. The more intense they are, the more pronounced will be the ecological responses of aquatic organisms that are bioindicators of water quality and may even exclude organisms sensitive to pollution Jaishankar et al. (2014), Outa et al. (2020)
Of particular concern are substances that are retained by organisms passing from one organism to another in the food chain. The result is possibly progressively higher concentrations of the chemical at higher levels of the food chain Ramos-Miras et al. (2019) To be able to predict the concentration of a substance in water from measured concentrations in aquatic organisms, it is important to know the impacts of substance discharges and toxic effects on these organisms Rowe (2014)
It has been widely observed that organisms (plankton, invertebrate, and vertebrate animals) can have high concentrations of certain contaminants about the concentrations of these substances in the environment in which they inhabit (air, water, soil, and sediment) Stankovic et al. (2014) This phenomenon is repeatedly called bioconcentration or bioaccumulation. Bioaccumulation is the process by which living beings absorb and retain chemical substances in their body; it can be directly through the environment that surrounds them (bioconcentration) and indirectly from food (biomagnification) Feng et al. (2020), Gecheva et al. (2020) This process involves several steps in the food chain and different types of food. As the trophic level increases, the greater will be the number of chemicals accumulated in the living being since, in addition to the compounds that its organism has already absorbed, it will also concentrate those that come from food Banerjee et al. (2015), Jothi et al. (2018)
Organisms that bioconcentrate or bioaccumulate substances can be explored with environmental contamination monitors. The wide use of fish is also explained by their ability to respond to toxic substances such as large vertebrates, thus being able to be bioindicators of carcinogenic and teratogenic potential in humans. Fish indicate the potential for exposing human populations to chemical genotoxic, being considered the major vectors of transfer of contaminants to humans Fakhri et al. (2020), Guo et al. (2016) Many toxic substances such as toxic elements and organic compounds can be transferred from the tissues of organisms to their predators and reach concentrations of greater magnitudes at higher trophic levels Salam et al. (2020), Kwok et al. (2014)
There are about twenty elements considered toxic to human health, including Al, Cr, Cu, Fe, Mn, Ni, Pb, and Zn, these are some of the elements with the greatest industrial use and, therefore, are the most studied from the point of view of from a toxicological point of view Nawab et al. (2018) Such elements react with diffusing ligands, with macromolecules, and with ligands present in membranes, which often give them the properties of bioaccumulation, biomagnification in the food chain, persistence in the environment, and disturbances in the metabolic process of living beings Fakhri et al. (2020), Mohammed et al. (2012) Bioaccumulation and biomagnifications are responsible for transforming concentrations considered normal into toxic concentrations for different species of biota and humans Lara et al. (2020) Persistence guarantees effects over time or the long term, even after emissions are stopped, thus transmitting the toxic effects of contaminants over time after contamination Yang et al. (2020), Cunningham et al. (2019)
Elements bioaccumulate in aquatic organisms of different shapes and sizes Nteziyaremye and Omara (2020), Viana et al. (2020), Yang et al. (2020), Vu et al. (2017) Bioaccumulation translates the accumulation of pollutants in organisms about the amount of pollutant present, respectively, in the soil, sediments, and water, being the sum of successive absorptions of a pollutant made directly, or through food, by aquatic species. This bioaccumulation phenomenon is well known due to the existence of certain species capable of accumulating significant amounts of natural substances Gobas et al. (2009), Banerjee et al. (2015)
In principle, a substance is considered bio accumulative when the BAF value exceeds a specific threshold. Depending on the regulatory framework, the bioaccumulation cut-off value ranges from 500 (not bio accumulative) to 5000 (very bio accumulative) Wassenaar et al. (2020)
According to Martins and Lima (2001) the bioaccumulation factor (BAF) of Mn, for example, can vary from 930 in fish to 20,000 in aquatic plants, which demonstrates the variability of the bioaccumulation factor of the metals inserted in the trophic chain.
In Brazil, the Amazon region has a large network of water resources, consisting of a large number of rivers, streams, and lakes, together with the large number of ichthyic species living in its waters. The Amazon has one of the largest hydrographic basins on the planet and it is a region that requires constant monitoring of the water quality of its rivers and special attention to the fish that are the basis of the diet of its population Barros et al. (2010), Pereira and Saraiva (2015)
Barcarena, a city located near Belém, capital of the state of Pará, is located in one of the largest alumina production industries in the world, which uses the Bayer process in production, generating a tailing known as red mud that is deposited in exposed sedimentation basins. The weather and which generates an effluent rich in toxic elements such as Al, Cr, Cu, Fe, Mn, Ni, Pb, and Zn. This effluent is discarded in the rivers of the region and the Murucupi river and the Pará river are the main rivers to receive these effluents. The fish caught in these rivers are consumed by the local population, who may be exposed to these elements with harmful health consequences.
2. METHODOLOGY
2.1. AREA OF STUDY
The municipality of Barcarena Figure 1 is located in the northeast of the state of Pará between the parallels 1º 30' S to 1º 40' S and between the meridians 48º 30' W to 48º 50' W. The municipality of Barcarena has a population of approximately 129,333 inhabitants, according to statistical data from the Ibge (2022) The main productive activities of the municipality are agriculture and industry. The municipality of Barcarena belongs to the Metropolitan mesoregion of Belém and the microregion of Belém.
Figure 1
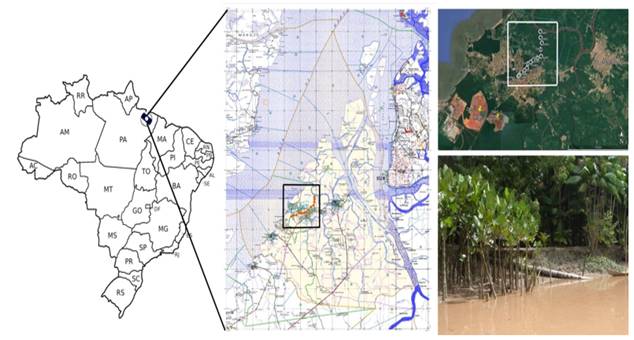
|
Figure 1 Illustrative map
showing the Murucupi river Source: Maps:
Adapted Ibge (2022) Google Earth (2022) |
The main bodies of water predominant in this region are constituted by the Marajó Bay, the Pará River, and the various rivers that communicate with each other through channels (called holes) and with the streams (streams of clear water). The largest rivers in the area are the Itaporanga, the Barcarena, and the Murucupi. Other important waterways are the Arienga river, Arrozal hole, the Dendê, Tauá, Pramajó, Pramajozinho, Água Verde, Japim, Pau Amarelo, Curuperê and Conde streams. In general, both the rivers and the streams and boreholes mentioned here, predominate the yellowish-brown colour of the waters Silva (2012)
The Murucupi River has its source located at 01º 33' 0.00” of south latitude and 48º 43' 0.00” of west longitude, within the area of an alumina industry, and its mouth at the coordinates of 01º 29' 31” of south latitude and 48º 40' 00.8” west longitude on the Barcarena River Silva (2012)
As a consequence of disorderly growth and the lack of sustainable growth planning, the contamination of water ecosystems has caused serious environmental impacts to the municipality and serious damage to the remaining communities on the banks of rivers and boreholes that cut the affected region. The city is currently experiencing serious environmental problems related not only to industries but also related to basic sanitation problems (dumps, domestic and sanitary sewers). Thus, natural waters as well as the geological environment as a whole, interact permanently, causing a series of chemical transformations.
2.2. COLLECTION AND TREATMENT OF SAMPLES
2.2.1. FISH
Twenty samples of the fish species Cichla spp (Tucunaré), Eigenmannia sp. (Ituí), and Angelfish (Acará) were collected along the Murucupi River in the rainy season (April). The samples, after pre-washing and biometrics, were cooled and taken to the laboratory, where tissue and gill aliquots were extracted and, after drying in an oven at 40 ºC, submitted to the microwave digestion process. The opening procedure followed the methodology described by the microwave manufacturer (Provecto). Around 0.3 g of dry mass was added to each reactor, and then the following reagents were added: 2 mL of Nitric Acid Suprapur 65% (Merck); 0.5 ml hydrogen peroxide 32% (Merck), then the vials were sealed and taken to the microwave oven.
The solutions resulting from this procedure were then measured with distilled water in a 50 mL volumetric flask for further elemental analysis. All water used had a minimum resistivity of 18.2 MΩ.cm-1 and was supplied by Elga's Purelab Ultra Analytic purification system, which used distilled water as a feed.
2.2.2. WATER
Fourteen samples of surface water were collected along the Murucupi River. Water collection was carried out with a 5-liter Van Dorn collection bottle, in the rainy season (April) when the intrusion of ocean waters into Marajó Bay is little pronounced in different tidal situations (high and low tide). The location of the water samples was georeferenced using a GPS (global positioning system).
Polyethylene flasks previously decontaminated with 10% nitric acid for 48 hours were used, which were washed with distilled water and ultrapure water, and at the place of collection, the environment was made with the sample itself. Two samples were collected from each point, one for testing the elements and the other for storage as a control, both were conditioned at 4 °C. The samples were filtered through GFF membranes (millipore 0.22 µm), for further analysis of the chemical elements. After filtration, the samples were acidified to pH < 2 with concentrated supra pure nitric acid.
2.3. METAL ANALYSIS AND ANALYTICAL QUALITY
The metals Al, Cr, Cu, Fe, Mn, Ni, Pb, and Zn were analysed using inductively coupled plasma optical emission spectrometry (ICP-OES) brand Varian Vista-PRO. The method presented a low detection and quantification limit and can be applied to matrices with metal levels in the µg.kg-1 range. An analytical quality control program (study of linearity, accuracy, and precision) was carried out using the NIST 1643e reference standard that was used to provide reliable data and calibrate the equipment for the river water matrix. The recoveries of the elements were between 76.25 - 105.91%. The DORM-2 reference material (dogfish muscle certified reference material) from the National Research Council was also used, where trace elements were determined in fish tissue. The results showed that the method adopted is suitable for the analysis of elements in fish with good accuracy and recoveries ranging from 90.92 to 109.30%.
2.4. STATISTIC
The programs used in descriptive statistics (mean, median, deviation, range) and multivariate (box plot, correlation, ANOVA) were Microsoft Excel and Statistica.
The Maximum Allowable Concentrations (MPC) in the tissue of the fish evaluated in this study were those recommended by ANVISA of the Ministry of Health (MS) of Brazil. The means of the results were compared with those found by other authors.
2.5. BIOACCUMULATION FACTOR (BAF)
This study followed the guidelines provided by USEPA (2000) using equation 1 to calculate the bioaccumulation factor (BAF).
BAF = FC/WC (1)
Where
FC represents the metal concentration in the evaluated fish species and
WC the concentration of the metal in the water of the river.
The maximum BAF values in freshwater fish tissue were
compared to that recommended by Karlsson et al. (2002), based on IAEA standards
364 and NCRP 123, for Al (500 L.kg-1), Cr (200 L.kg-1),
Cu (200 L.kg-1), Fe (200 L.kg-1), Mn (400-500 L.kg-1),
Ni (100 L.kg-1), Pb (300 L.kg-1) and Zn (1000 L.kg-1).
3. RESULTS AND DISCUSSION
3.1. FISH
The results of the quantification of tissue and gill elements of fish species collected in the Murucupi River are described in Table 1
Brazilian legislation Anvisa (2021) does not establish concentration limits in fish tissue for the elements evaluated in this study, except for Pb (0.30 mg.kg-1), which was in non-compliance in all species evaluated, presenting a general average of 0.442±0.128 mg.kg-1, around two times higher than the maximum value allowed, with a concentration range varying from 0.250-0.680 mg.kg-1.
Table 1
|
Table 1 Descriptive Statistics of the concentration of metals in tissue and gill of fish by species (mg.kg-1) |
||||||||
|
Al |
Cr |
Cu |
Fe |
Mn |
Ni |
Pb |
Zn |
|
|
RDC
Nº 4871 |
- |
- |
- |
- |
- |
- |
0.3 |
- |
|
Tissue |
||||||||
|
Cichla
spp |
||||||||
|
Mean |
82.9 |
0.061 |
22.6 |
9.76 |
1.18 |
0.6 |
0.363 |
219 |
|
Median |
4.84 |
0.048 |
20.9 |
1.48 |
0.045 |
0.611 |
0.36 |
213 |
|
Standard
deviation |
158 |
0.037 |
15.1 |
16.7 |
2.28 |
0.304 |
0.124 |
74.5 |
|
Minimum |
2.39 |
0.033 |
8.37 |
1.29 |
0.032 |
0.283 |
0.25 |
136 |
|
Maximum |
320 |
0.116 |
40.3 |
34.8 |
4.61 |
0.894 |
0.48 |
312 |
|
Eigenmannia
sp |
||||||||
|
Mean |
4.07 |
0.047 |
57.7 |
1.33 |
7.92 |
0.579 |
0.42 |
184 |
|
Median |
4.07 |
0.047 |
57.7 |
1.33 |
7.92 |
0.579 |
0.42 |
184 |
|
Standard
deviation |
0.867 |
0.035 |
15.3 |
0.849 |
11.1 |
0.657 |
0.099 |
10.6 |
|
Minimum |
3.46 |
0.022 |
46.8 |
0.727 |
0.041 |
0.114 |
0.35 |
176 |
|
Maximum |
4.69 |
0.071 |
68.5 |
1.93 |
15.8 |
1 |
0.49 |
191 |
|
Angelfish |
||||||||
|
Mean |
181 |
0.033 |
32.8 |
27.4 |
4.32 |
0.257 |
0.533 |
413 |
|
Median |
193 |
0.035 |
36 |
25.1 |
5.28 |
0.282 |
0.5 |
457 |
|
Standard
deviation |
180 |
0.012 |
18.4 |
25.9 |
3.03 |
0.069 |
0.102 |
162 |
|
Minimum |
0.478 |
0.017 |
8.58 |
1.8 |
0.002 |
0.156 |
0.45 |
182 |
|
Maximum |
340 |
0.047 |
50.8 |
57.8 |
6.74 |
0.309 |
0.68 |
557 |
|
Gill |
||||||||
|
Cichla
spp |
||||||||
|
Mean |
222 |
0.04 |
25.2 |
29.9 |
2.69 |
0.389 |
0.332 |
172 |
|
Median |
279 |
0.04 |
24.9 |
30.8 |
2.52 |
0.342 |
0.315 |
164 |
|
Standard
deviation |
152 |
0.005 |
16 |
21.9 |
2.2 |
0.117 |
0.105 |
89.5 |
|
Minimum |
1.47 |
0.034 |
7.89 |
2.2 |
0.182 |
0.311 |
0.226 |
83 |
|
Maximum |
329 |
0.045 |
43 |
55.7 |
5.54 |
0.562 |
0.47 |
278 |
|
Eigenmannia
sp. |
||||||||
|
Mean |
219 |
0.064 |
28.7 |
36.8 |
5.62 |
0.327 |
0.53 |
378 |
|
Median |
219 |
0.064 |
28.7 |
36.8 |
5.62 |
0.327 |
0.53 |
378 |
|
Standard
deviation |
236 |
0.009 |
3.21 |
3.54 |
1 |
0.107 |
0 |
65.8 |
|
Minimum |
51.4 |
0.057 |
26.4 |
34.3 |
4.91 |
0.251 |
0.53 |
331 |
|
Maximum |
386 |
0.07 |
31 |
39.3 |
6.33 |
0.402 |
0.53 |
424 |
|
Angelfish |
||||||||
|
Mean |
5.14 |
0.064 |
32.8 |
4.05 |
0.133 |
0.681 |
0.256 |
60.5 |
|
Median |
4.93 |
0.061 |
33.1 |
4.66 |
0.125 |
0.709 |
0.257 |
65.5 |
|
Standard
deviation |
3.25 |
0.039 |
21.4 |
1.4 |
0.024 |
0.207 |
0.047 |
49.9 |
|
Minimum |
1.49 |
0.029 |
8.72 |
1.97 |
0.116 |
0.402 |
0.214 |
7 |
|
Maximum |
9.22 |
0.105 |
56.4 |
4.91 |
0.166 |
0.902 |
0.298 |
104 |
|
Anvisa (2021) Cichla spp (Tucunaré); Eigenmannia sp.
(Ituí); Angelfish (Acará); in bold are Pb concentrations in non-compliance
with legislation |
||||||||
The toxic effects of Pb in the human organism include disturbances in the nervous system, it causes a disease called saturnism, which causes problems in the nervous system causing irritation, dementia, madness, and can lead to death. It also causes anemia, cardiovascular disease, disturbances in bone metabolism, kidney function, and reproduction, and can cause cancer and lead to death. Atsdr (2005)
The Cichla spp species presented the highest concentrations of Cr and Ni, with little variation about. The Eigenmannia sp species and significant variation, around twice as high, concerning the Angelfish species. The species Eigenmannia sp had the highest concentrations of Cu and Mn, around twice as high as the other species.
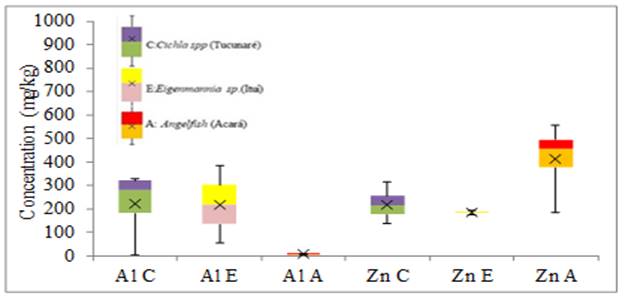
Al, Fe, Pb, and Zn showed the highest concentrations in Angelfish tissue with a significant increase among the other species for Al around two times greater than the species Cichla spp and forty-four times greater than the species Eigenmannia sp, Fe around twenty times higher in Eigenmannia sp and twice concerning Cichla spp.
Pb presented a small increase with the other species and Zn, presented an increase around twice for the two other species studied.
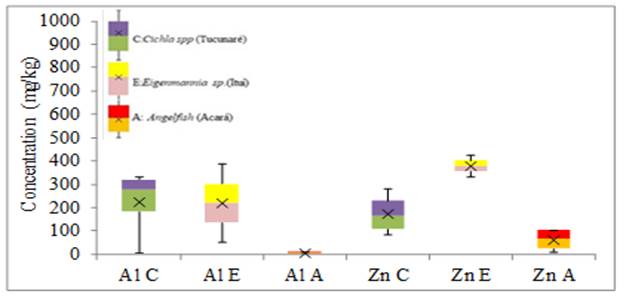
The species Cichla spp presented the highest concentrations of Al in the gills, concerning the species Eigenmannia sp. the increase was very small, while with the Angelfish species the increase was forty-three times. Cr was found in higher concentrations in Eigenmannia sp., and Angelfish with the same concentration, concerning the species Cichla spp the concentration had an increase around two times.
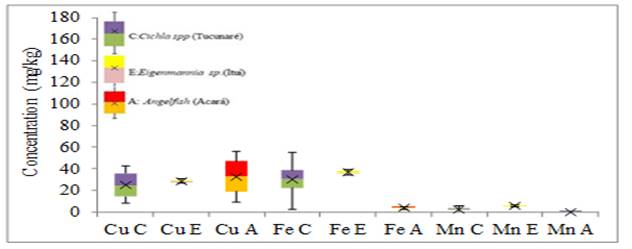
The Angelfish species presented the highest concentrations of Cu, with a small increase from the other species. The Eigenmannia sp. species presented the highest Fe elevation with a small elevation about the Cichla spp species and nine times higher than the Fe found in the Angelfish species. The concentration of Mn in the gills was higher in the Eigenmannia sp. with a twofold increase with the Cichla spp species and forty-two times in the Angelfish species. The Angelfish species presented the highest Ni concentrations, with a two-fold increase over the other species. The species Eigenmannia sp. presented the highest concentrations of Pb and Zn with increases of two times the concentration of Pb of the other species and for Zn, the increase was of two times to the species Cichla spp and six times concerning the species Angelfish.
The variability of the results of the concentrations of the elements studied is shown in Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 (Box plot).
For the minor elements (Cr, Pb, and Mn) the greatest variability of results in tissue and gills was for Ni in Eigenmannia sp. and Angelfish respectively, without anomalous and extreme results. For the elements Cu, Fe and Mn the greatest variability of results of metals in the tissues was for the Fe of the Angelfish species, in the gills the greatest variability was also of the Fe found in the Cichla spp species without anomalous and extreme results. For the largest elements in the fish tissue, the greatest variability of results was for Al in the Angelfish species without anomalous or extreme results. As for the gills, the greatest variability of results was for Al in the species Eigenmannia sp. without anomalous and extreme results. Anomalous results were found for Cr, Fe, and Mn in the tissue of the Cichla spp. No extreme results were obtained in this study.
Differences in aquatic environments regarding the type and level of pollution in the water, chemical form of the metal in the water, temperature, pH, dissolved oxygen concentration, and transparency, are factors that influence the concentration of toxic elements in different species of fish. There are also reports that geographic locations and seasonal variation contribute to different concentrations of metals, even in the same fish species Dural et al. (2007), Bahnasa et al. (2009)
The Murucupi River is directly affected by the tidal variation, which causes an intense variation in the physical-chemical properties of the water body and probably, directly interferes with the concentration of available metals in the water column, contributing to the absorption of these elements by the fish.
Mccarthy and Shugart (1990) state that fish ingest toxic elements in their food and surrounding water, and this leads to the accumulation of these elements in significant amounts in various tissues. This suggests that the metals determined in the fish from the Murucupi River may have their origin in these sources.
Figure 2
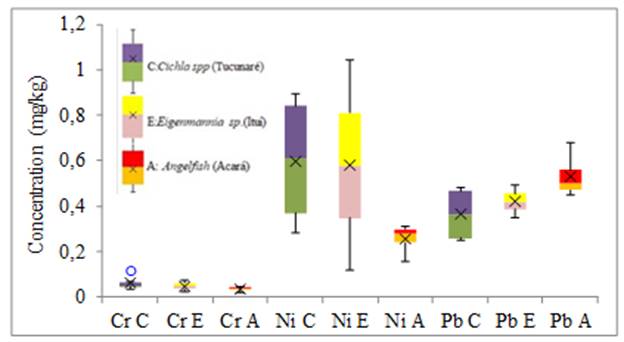
|
Figure 2 Variability in the concentration of Cr, Ni and Pb elements in fish tissue (mg.kg-1) |
Figure 3
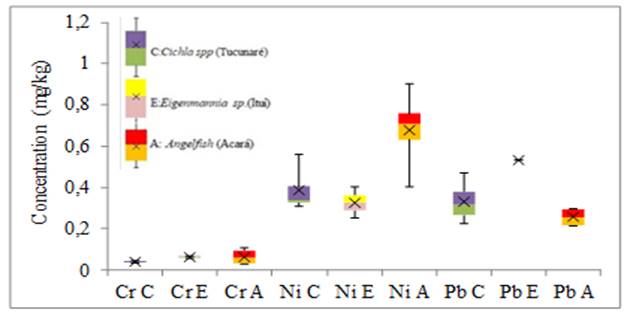
|
Figure 3 Variability in the concentration of Cr, Ni and Pb elements in fish gills (mg.kg-1) |
Figure 4
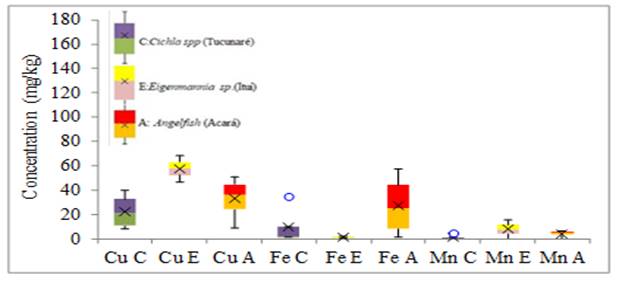
|
Figure 4 Variability in the concentration of Cu, Fe and Mn elements in fish tissue (mg.kg-1) |
Figure 5
|
Figure 5 Variability of the concentration of Cu, Fe and Mn elements in fish gills (mg.kg-1) |
Figure 6
|
Figure 6 Variability in the concentration of Al and Zn elements in fish tissue (mg.kg-1) |
Figure 7
|
Figure 7 Variability in the concentration of Al and Zn elements in fish gills (mg.kg-1) |
Comparing the results found for the elements found by other authors Table 2 it was found that the average concentration of Al in tissue was one and a half times higher than the average found by other authors in the Cichla spp species and three times higher in the species Angelfish, for the species Eigenmannia sp the Al in this study showed a concentration fourteen times lower than that found by other authors.
The average concentration of Cu in tissue was twenty-four times higher than the average found by other authors in the Cichla spp species, sixty-two times higher in the Eigenmannia sp. and thirty-five times higher in the Angelfish species. The average concentration of Mn in tissue was 1.4 times higher in the Cichla spp species, nine times higher in the Eigenmannia sp., and five times higher in the Angelfish species.
The average concentration of Zn in tissue was forty-nine times higher in the Cichla spp species, forty-one times higher in the Eigenmannia sp., and ninety-three times greater in the Angelfish species, which was the highest increase presented in comparison to the averages found by other authors.
For Pb, only the average for the Angelfish species was 1.07 times higher than that found by other authors. For Cr, Fe, and Ni, all averages found were lower when compared to other works.
Table 2
|
Table 2 Results of the concentration of elements in fish tissue (mg.kg-1) |
|||||||||
|
Species |
Al |
Cr |
Cu |
Fe |
Mn |
Ni |
Pb |
Zn |
Reference |
|
CA |
- |
0.19 |
0.934 |
- |
- |
- |
0.811 |
6.45 |
Yi and Zhang (2012) |
|
CC |
- |
0.239 |
0.99 |
- |
- |
- |
0.43 |
5 |
Yi and Zhang (2012) |
|
CH |
- |
0.123 |
0.97 |
- |
- |
- |
0.53 |
3.45 |
Yi and Zhang (2012) |
|
CI |
- |
0.121 |
0.834 |
- |
- |
- |
0.21 |
2.8 |
Yi and Zhang (2012) |
|
CR,
HN, OP |
- |
7.02 |
2.97 |
64.2 |
- |
0.97 |
1.36 |
- |
Jothi et al. (2018) |
|
CS |
46.1 |
1.45 |
- |
- |
- |
- |
- |
- |
Barros et al. (2010) |
|
CS,
PM, PSq, SS |
8.59 |
- |
- |
11.5 |
1.73 |
- |
- |
5.12 |
Pereira and Saraiva (2015) |
|
HM |
- |
0.206 |
0.771 |
- |
- |
- |
0.529 |
3.39 |
Yi and Zhang (2012) |
|
MC |
- |
- |
0.907 |
- |
0.834 |
0.978 |
0.172 |
12.8 |
Elnabris et al. (2013) |
|
MF |
- |
- |
0.345 |
- |
0.396 |
0.453 |
0.552 |
20.5 |
Elnabris et al. (2013) |
|
MH |
- |
- |
0.318 |
- |
0.519 |
0.707 |
<LOQ |
5.82 |
Elnabris et al. (2013) |
|
PS |
124 |
1.75 |
- |
- |
- |
- |
- |
- |
Barros et al. (2010) |
|
SA |
- |
0.209 |
0.78 |
- |
- |
- |
0.55 |
4.6 |
Yi and Zhang (2012) |
|
SS |
46.1 |
0.85 |
- |
- |
- |
- |
- |
- |
Barros et al. (2010) |
|
General
mean |
56.2 |
0.168 |
0.932 |
37.9 |
0.87 |
0.777 |
0.495 |
4.43 |
|
|
Bagre - Silurusasotus (SA), Bombay duck
- Harpadon nehereus (HN), Branquinha comum - Potamorhina spp (PS), Carpa
prateada - Hypophthalmichthys molitrix (HM), Carpa capim -
Ctenopharyngodonidellus(CI), Carpa comum -Cyprinuscarpio (CC), Coreius -
Coreius heterodom (CH), Corvina - Micropogonias furnieri (MF), Merluza -
Merluccius hubbsi (MH), Pacu - Piaractus mesopotamicus (PM), Pama croaker -
Otolithoides pama (OP), Pescada - Plagioscion squamosissimus (PSq), Pimpão-
Carassius auratus(CA), Piranha - Serrasalmus spp (SS), Rat-tail anchovy -
Coilia ramcarati (CR), Tainha - Mugil cephalus (MC), Tucunaré - Cichla ssp
(CS). <LOQ = below the quantification limit of the method |
|||||||||
3.2. CORRELATION
Few correlations were found between the chemical elements in the tissue of the evaluated fish species. An excellent significant positive correlation (p < 0.05) was found between Al and Fe (r = 0.968, p = 0.000), indicating that these elements may have the same origin, probably because they are natural constituents of Amazonian geochemistry and are macroconstituents of the chemical composition of red mud, which is the waste from alumina production. Significant good negative correlations (p < 0.05) were also found in fish tissue between Mn and Ni (r = -0.661, p = 0.037) and between Ni and Pb (r = -0.772, p = 0.009).
In the gills, significant positive correlations (p < 0.05) were found between Al and Fe (r = 0.801, p = 0.005), between Fe and Mn (r = 0.752, p = 0.012), Fe and Pb (r = 0.824, p = 0.003) and Fe and Zn (r = 0.654, p = 0.040), Mn and Pb (r = 0.732, p = 0.016), Mn and Zn (r = 0.687, p = 0.028), Pb and Zn (r = 0.868, p = 0.001) showing that, unlike the tissue, the gills are directly influenced by exposure to elements present in the aquatic ecosystem. Significant negative correlations (p < 0.05) between Fe and Ni (r = -0.780, p = 0.008), Mn and Ni (r = -0.732, p = 0.016), Ni and Pb (r = -0.702, p = 0.024), Ni and Zn (r = -0.649, p = 0.042).
3.3. DIFFERENCE BETWEEN SPECIES
To verify if the means between the concentrations of the elements differed significantly in the tissue and in the gills of the evaluated species, the simple Anova test was used. This study showed that in the tissues the elements did not differ significantly, showing that the species does not constitute a determining factor in the variation of the concentration of metals present in the tissues.
As for the gills, the means between the evaluated elements were different for the Mn where the Fcalculated (9.36) was greater than the Fcritical (4.74) with a significance of 0.011. The means also differed significantly for Pb (Fcalculated (8.81) > Fcritical (4.74) p = 0.012) and for Zn (Fcalculated (13.11) > Fcritical (4.74) p = 0.004). For the gills, the species influenced the concentration of metals found in the species evaluated, proving that the gills, which function as filters, absorb elements according to the environment, the habits, and characteristics of the species.
3.4. WATER
The results for the concentration of elements in the water of the Murucupi River are shown in Table 3 The elements that did not comply with the legislation adopted in Brazil were Al, Cu and Fe. Al was around fifty-seven times above the maximum limit recommended by legislation. Cu was twice the maximum allowed and Fe was around three times the maximum allowed.
Table 3
|
Table 3 Descriptive statistics of the concentration of chemical elements in the water of the Murucupi River (mg. L-1) |
||||||||
|
Al |
Cr |
Cu |
Fe |
Mn |
Ni |
Pb |
Zn |
|
|
RES 357/051 |
0.1 |
0.05 |
0.009 |
0.3 |
0.1 |
0.025 |
0.01 |
0.18 |
|
Mean |
5.66 |
0.007 |
0.02 |
0.775 |
0.058 |
0.004 |
0.008 |
0.052 |
|
Median |
6.03 |
0.007 |
0.016 |
0.736 |
0.066 |
0.005 |
0.008 |
0.053 |
|
Standard deviation |
1.63 |
0.001 |
0.009 |
0.14 |
0.022 |
0.002 |
0.004 |
0.017 |
|
Minimum |
1.92 |
0.006 |
0.012 |
0.601 |
0.018 |
<LOQ |
0.004 |
0.025 |
|
Maximum |
7.39 |
0.01 |
0.038 |
1.04 |
0.079 |
0.007 |
0.016 |
0.091 |
|
Conama (2005) Results in bold are in non-compliance
with the CONAMA resolution (RES) 357/05; <LOQ = below the quantification
limit of the method |
||||||||
The Al found in this study had a sixteen-fold increase when compared to the Al found by Pereira et al. (2007) the average concentration of 0.356 mg. L-1, in a normal situation without effluent leakage. The high average values for Al in the water of the Murucupi River at the time of the red mud effluent leak show the relationship of this element with the effluent rich in this metal. Fe and Cu are also constituent elements of red mud, present in high concentrations in bauxite and in the waste generated by its processing to obtain calcined alumina.
Pereira et al. (2007) report the importance of concern with these results, justifying that the Murucupi River flows into the Pará River, an important river in the region, where the pollution plume is dissipated, reaching the edge of the city of Belém and islands under the area of influence of the Pará river basin, where many families use this water without treatment for consumption, fishing and bathing, thus making the problem of water pollution a public health issue.
3.5. BIOACCUMULATION FACTOR (BAF) OF ELEMENTS IN THE TISSUE AND GILLS OF FISH
The bioaccumulation factor (BAF) of the elements evaluated in the tissue and gills of the fish species evaluated in this study is described in Table 4
The bioaccumulation followed the following order in the tissue of the Cichla spp species Zn > Cu > Ni > Pb > Mn > Al > Fe > Cr in the gills the order was Zn > Cu > Ni > Mn > Pb > Al > Fe > Cr. In the tissue of the Eigenmannia sp. the order was Zn > Cu > Ni > Mn > Pb > Cr > Fe > Al in the gills the order was Zn > Cu > Mn > Ni > Pb > Fe > Al > Cr. In Angelfish tissue the order was Zn > Cu > Mn > Pb > Ni > Fe > Al > Cr in the gills the order was Cu > Zn > Ni > Pb > Cr > Fe > Mn > Al.
Table 4
|
Table 4 Bioaccumulation factor of elements in fish collected in the Murucupi River (tissue and gill) (L.kg 1) |
||||||||
|
BAF |
Al |
Cr |
Cu |
Fe |
Mn |
Ni |
Pb |
Zn |
|
Cichla spp |
||||||||
|
BAF tissue |
14.6 |
8.71 |
1130 |
12.59 |
20.3 |
150 |
45.4 |
4212 |
|
BAF gill |
39.2 |
5.71 |
1260 |
38.6 |
46.4 |
97.3 |
41.5 |
3308 |
|
Eigenmannia sp. |
||||||||
|
BAF tissue |
0.719 |
6.71 |
2885 |
1.72 |
137 |
145 |
52.5 |
3538 |
|
BAF gill |
38.7 |
9.14 |
1435 |
47.5 |
96.9 |
81.8 |
66.3 |
7269 |
|
Angelfish |
||||||||
|
BAF tissue |
32 |
4.71 |
1640 |
35.4 |
74.5 |
64.3 |
66.6 |
7942 |
|
BAF gill |
0.908 |
9.14 |
1640 |
5.23 |
2.29 |
170 |
32 |
1163 |
|
BAF values in bold
are above those recommended by Karlsson et al., 2002 |
||||||||
Among the species studied, Angelfish presented the highest BAF for Zn in the tissues, for Cu the species that presented the highest BAF was Eigenmannia sp. In the gills, the species that presented the highest BAF for Zn was Eigenmannia sp., for Cu it was Angelfish.
According to Karlsson et al. (2002) only the elements Cu (BAF around seven times higher in the Cichla spp species, fourteen times higher in the Eigenmannia sp. and eight times higher in the Angelfish species), Ni (BAF of one and a half times higher in the Cichla spp and Eigenmannia sp. species) and Zn, (BAF around four times higher in the Cichla spp species, four times higher in the Eigenmannia sp. and eight times higher in the Angelfish species), showed BAF values above the recommended. The elements Al, Cr, Fe, Mn and Pb did not present significant BAF.
By analysing the results of the BAF in the samples of fish collected in the Murucupi River, it is observed that there is a non-linear variation in the bioaccumulation of metals in the species of fish collected, which may be related to the fact that the high amount of material in suspension in the bed of the Murucupi river at the time of the environmental accident. The suspended material may have been incorporated into organisms in a diversified way, raising the levels of some elements in the tissues and gills of the fish collected.
Regarding the effects of the bioaccumulation of metals in the evaluated fish, it was observed that Cu is one of the most toxic metals for fish, Cu cause a rapid loss of electrolytes Lloyd (1992) With the depletion of salts in the blood, the water present in the plasma flows to the tissues causing an increase in viscosity due to a massive hemoconcentration. According to Wilson and Taylor (1993) the heart fails to deal with highly viscous blood.
Ni is carcinogenic and mutagenic. Some observed effects of Ni in aquatic environments include tissue damage to organisms, genotoxicity, and reduced growth rates Dallas et al. (2013)
Zn is an element that, when accumulated in large quantities in the fish organism, is capable of causing histopathological changes in the gills such as hyperplasia, lamellar fusion, epithelial destruction, and excessive mucus production Hogstrand et al. (1994) Santos (2009) in addition to immunotoxic effects Mottin et al. (2010) Furthermore, it is capable of causing a decrease in food consumption Kuz'mina (2011)
4. CONCLUSIONS
The species composition and temporal distribution of aquatic organisms are altered by the actions of impacts. The more intense they are, the more pronounced the ecological responses of aquatic organisms that are bioindicators of water quality will be.
The study suggests that human activities are possible sources of pollution by toxic elements in the Murucupi River and the fish species examined can be used to monitor the levels of Al, Cr, Cu, Fe, Mn, Ni, Pb and Zn in the river. Within this perspective, it is considered that certain groups of aquatic organisms, such as fish, can be useful as bioindicators, not only from an academic point of view, but also for making political decisions about environmental preservation. These results reinforce the affirmation of the anthropic contribution in the Murucupi River of the tailings of the bauxite beneficiation process.
ACKNOWLEDGEMENTS
The authors thank the support of the Environmental Police Department (Civil Police - State of Pará), the Central Laboratory of the Secretariat of Health of the State of Pará, the National Research Council, the Federal University of Pará, and Barcarena community leaders for the all support.
REFERENCES
Anvisa. (2021). Agência Nacional de Vigilância Sanitária - Ministério da Saúde. Resolução RDC Nº 487, Dispõe sobre os limites máximos tolerados (LMT) de contaminantes em alimentos.
Atsdr. (2005). Agency for Toxic Substances and Disease Registry. Toxicological Profile for Lead. Division of Toxicology and Environmental Medicine. Atlanta, Georgia.
Bahnasa, W. Y. M. Khidr, A. Dheina, N. (2009). Seasonal Variations of Heavy Metals Concentrations in Mullet, Mugil Cephalus and Liza Ramada (Mugilidae) from Lake Manzala, Egypt, 13(2), 81-100. https://doi.org/10.21608/ejabf.2009.2034
Banerjee, S. Maiti, S. K. Kumar, A. (2015). Metal Contamination in Water and Bioaccumulation of Metals in the Planktons, Molluscs and Fishes in Jamshedpur Stretch of Subarnarekha River of Chotanagpur Plateau, India. Water and Environment Journal, 29(2), 207-213. https://doi.org/10.1111/wej.12108
Barros, B. C. Pereira, S. F. P. Palheta, D. C. Silva, C. S. (2010). Determinação de Cd, Cr e Al em Tecido de Peixes Provenientes do Rio Gelado/APA, Floresta de Carajás-PA. Holos Environment, 10(2), 195-208. https://doi.org/10.14295/holos.v10i2.3668
Conama. (2005). Conselho Nacional de Meio Ambiente. Resolução n 357 de 17 de março de.
Cunningham, P. A. Sullivan, E. E. Everett, K. H. Kovach, S. S. Rajan, A. Barber, M. C. (2019). Assessment of Metal Contamination in Arabian/Persian Gulf Fish : A Review. Marine Pollution Bulletin, 143, 264-283. https://doi.org/10.1016/j.marpolbul.2019.04.007
Dallas, L. J. Bean, T. P. Turner, A. Lyons, B. P. Jha, A. N. (2013). Oxidative DNA Damage May Not Mediate Ni-Induced Genotoxicity in Marine Mussels : Assessment of Genotoxic Biomarkers and Transcriptional Responses of Key Stress Genes. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 754(1-2), 22-31. https://doi.org/10.1016/j.mrgentox.2013.03.009
Dural, M. Göksu, M. Z. L. Özak, A. A. (2007). Investigation of Heavy Metal Levels in Economically Important Fish Species Captured from the Tuzla Lagoon. Food Chem, 102(1). 415-421. https://doi.org/10.1016/j.foodchem.2006.03.001
Elnabris, K. J. Muzyed, S. K. El-Ashgar, N. M. (2013). Heavy Metal Concentrations in Some Commercially Important Fishes and Their Contribution to Heavy Metals Exposure in Palestinian People of Gaza Strip (Palestine). Journal of the Association of Arab Universities for Basic and Applied Sciences, 13(1), 44-51. https://doi.org/10.1016/j.jaubas.2012.06.001
Fakhri, Y. Djahed, B. Toolabi, A. Raoofi, A. Gholizadeh, A. Eslami, H. Taghavi, M. Alipour, M. R. Khaneghah, A. M. (2020). Potentially Toxic Elements (Ptes) in Fillet Tissue of Common Carp (Cyprinus Carpio) : A Systematic Review, Meta-Analysis and Risk Assessment Study. Toxin Reviews, 40(4), 1505-1517. https://doi.org/10.1080/15569543.2020.1737826
Feng, W. Wang, Z. Xu, H. Zhang, D. Zhang, H. Zhu, W. (2020). Species Specifc Bioaccumulation of Trace Metals Among Fsh Species From Xincun Lagoon. Scientifc Reports, 1-11. https://doi.org/10.1038/s41598-020-77917-y
Gecheva, G. Yancheva, V. Velcheva, I. Georgieva, E. Stoyanova, S. Arnaudova, D. Stefanova, V. Georgieva, D. Genina, V. Todorova, B. Mollov, I. (2020). Integrated Monitoring with Moss-Bag and Mussel Transplants in Reservoirs. Water, 12(6), 1-19. https://doi.org/10.3390/w12061800
Gobas, F. A. P. C. Wolf, W. Burkhard, L. P. Verbruggen, E. Plotzke, K. (2009). Revisiting Bioaccumulation Criteria for POPs and PBT Assessments. Integrated Environmental Assessment and Management, 5(4), 624-637. https://doi.org/10.1897/IEAM_2008-089.1
Google Earth. (2022). Programa de Mapas Por Satélite. Disponível em.
Guo, B. Jiao, D. Wang, J. Lei, K. Lin, C. (2016). Trophic Transfer of Toxic Elements in the Estuarine Invertebrate and Fish Food Web of Daliao River, Liaodong Bay, China. Marine Pollution Bulletin, 113(1-2), 258-265. https://doi.org/10.1016/j.marpolbul.2016.09.031
Hogstrand, C. Wilson, R. W. Polgar, D. Wood, C. M. (1994). Effects of Zinc on the Kinetics of Branchial Calcium Uptake in Freshwater Rainbow Trout During Adaptation to Waterbone Zinc. J. Exp. Biol, 186(1), 55-73. https://doi.org/10.1242/jeb.186.1.55
Ibge. (2022). Instituto Brasileiro De Geografia E Estatística. Dados Da População Do Município De Barcarena-PA Disponível Em.
Jaishankar, M. Tseten, T. Anbalagan, N. Mathew, B. B. Beeregowda, K. N. (2014). Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol, 7(2), 60-72. https://doi.org/10.2478/intox-2014-0009
Jothi, J. S. Anka, I. Z. Hashem, S. Morshed, S. (2018). Assessment of Heavy Metal Concentration in Edible Fish Muscle and Water Sample Collected from Different Location In Chittagong : A Public Health Concern. Ukrainian Food Journal, 7(3), 464-471. https://doi.org/10.24263/2304-974X-2018-7-3-11
Karlsson, S. Meili, M. Studsvik, U. B. (2002). Bioaccumulation Factors in Aquatic Ecosystems : A Critical Review. Swedish Nuclear Fuel And Waste Management Co, 67.
Khaneghaha, A. M. Sant'ana, A. S. (2020). Systematic Review and Meta-Analysis: Applications in Food Science, Challenges, and Perspectives. Food Research International, 134, 109245. https://doi.org/10.1016/j.foodres.2020.109245
Kumari, P. (2018). Distribution of Metal Elements in Capillary Water, Overlying Water, Sediment, and Aquatic Biota of Three Interconnected Ecosystems. Environ. Process, 5, 385-411. https://doi.org/10.1007/s40710-018-0303-x
Kuz'mina V. V. (2011). The Influence of Zinc and Copper on the Latency Period for Feeding and the Food Uptake in Common Carp, Cyprinus Carpio L. Aquatic Toxicol, 102(1-2), 73-78. https://doi.org/10.1016/j.aquatox.2010.12.018
Kwok, C. K. Liang, Y. Wang, H. Dong, Y. H. Leung, S. Y. Wong, M. H. (2014). Bioaccumulation of Heavy Metals in Fish and Ardeid At Pearl River Estuary, China. Ecotoxicology and Environmental Safety, 106, 62-67. https://doi.org/10.1016/j.ecoenv.2014.04.016
Lara, A. Galván-Magaña, F. Elorriaga-Verplancken, F. Marmolejo-Rodríguez, A. J. Gonzalez-Armas, R. Arreola-Mendoza, L. Sujitha, S. B. Jonathan, M. P. (2020). Bioaccumulation and Trophic Transfer of Potentially Toxic Elements in the Pelagic Thresher Shark Alopias Pelagicus in Baja California Sur, Mexico. Marine Pollution Bulletin, 156, 1-9. https://doi.org/10.1016/j.marpolbul.2020.111192
Lloyd, R. (1992). Pollution and Freshwater Fish. The Buckland Foundation, Oxford. 16, 176.
Martins, I. Lima, I. V. (2001). Ecotoxicologia Do Manganês E Seus Compostos. Salvador : Centro De Recursos Ambientais, Série Cadernos De Referência Ambiental, 7, 121.
Mccarthy, J. Shugart, L. (1990). Biomarkers of Environmental Contamination. Lewis Publishers, CRC Press, New York, 73.
Mohammed, A. May, T. Echols, K. Walther, M. Manoo, A. Maraj, D. Agard, J. Orazio, C. (2012). Metals in sediments and fish from Sea Lots and Point Lisas harbors, Trinidad and Tobago. Marine Pollution Bulletin, 64(1), 169-173. https://doi.org/10.1016/j.marpolbul.2011.10.036
Mottin, E. Caplat, C. Mahaut, M. L. Costil, K. Barillier, D. Lebel, J. M. Serpentini, A. (2010). Effect of in Vitro Exposure to Zinco on Immunological Parameters of Haemocytes from the Marine Gastropod Haliotis Tuberculata. Fish & Shellfish Immunology, 29(5), 846-853. https://doi.org/10.1016/j.fsi.2010.07.022
Nawab, J. Khan, S. Xiaoping, W. (2018). Ecological and Health Risk Assessment of Potentially Toxic Elements in the Major Rivers of Pakistan : General Population Vs. Fishermen. Chemosphere, 202, 154-164. https://doi.org/10.1016/j.chemosphere.2018.03.082
Nteziyaremye, P. Omara, T. (2020). Bioaccumulation of Priority Trace Metals in Edible Muscles of West African Lungfish (Protopterus Annectens Owen, 1839) from Nyabarongo River, Rwanda. Cogent Environmental Science, 6(1), 1-16. https://doi.org/10.1080/23311843.2020.1779557
Omar, W. A. Wafai, M. Z. A. Abdo, H. M. Defan, T. A. A. E. Poraas, M. M. (2015). Ecological Risk Assessment of Metal Pollution along Greater Cairo Sector of the River Nile, Egypt, Using Nile Tilapia, Oreochromis niloticus, as Bioindicator. Journal of Toxicology, 1-11. https://doi.org/10.1155/2015/167319
Outa, J. O. Kowenje, C. O. Avenant-Oldewage, A. Jirsa, F. (2020). Trace Elements in Crustaceans, Mollusks and Fish in the Kenyan Part of Lake Victoria : Bioaccumulation, Bioindication and Health Risk Analysis. Archives of Environmental Contamination and Toxicology, 78, 589-603. https://doi.org/10.1007/s00244-020-00715-0
Pereira, S. F P. Lima, M. A. Freitas, K. H. Mescouto, C. S. Saraiva, A. F. (2007). Estudo Químico Ambiental Do Rio Murucupi - Barcarena, PA, Brasil, Area Impactada Pela Produção De Alumínio. Ambi-Agua, Taubaté, 2(3), 62-82. https://doi.org/10.4136/ambi-agua.34
Pereira, S. F. P. Saraiva, A. C. F. (2015). Avaliação De Elementos Tóxicos Em Agua, Cabelo E Peixe Na Região Da Volta Grande Do Rio Xingu - Local Da Futura UHE Belo Monte. VIII Congresso de Inovação Tecnológica em Energia Elétrica (CITENEL), Costa do Sauípe-BA, 1-8.
Ramos-Miras, J. J. Sanchez-Muros, M. J. Morote, E. Torrijos, M. Gil, C. Zamani-Ahmadmahmoodi, R. Martin, J. A. R. (2019). Potentially Toxic Elements in Commonly Consumed Fish Species from the Western Mediterranean Sea (Almería Bay) : Bioaccumulation in Liver and Muscle Tissues in Relation to Biometric Parameters. Science of the Total Environment, 671, 280-287. https://doi.org/10.1016/j.scitotenv.2019.03.359
Rowe, C. L. (2014). Bioaccumulation and Effects of Metals and Trace Elements from Aquatic Disposal of Coal Combustion Residues: Recent Advances and Recommendations for Further Study. Science of the Total Environment, 490-496. https://doi.org/10.1016/j.scitotenv.2014.03.119
Salam, M. A. Paul, S. C. Zain, R. A. M. Bhowmik, M. S. Nath, M. R. Siddiqua, S. A. Aka, T. D. Iqbal, M. A. Kadir, W. R. Ahamad, R. B. Khaleque, A. Rak, A. E. Amin, M. F. M. (2020). Trace Metals Contamination Potential and Health Risk Assessment of Commonly Consumed Fish of Perak River, Malaysia. Plos One, 26, 1-18. https://doi.org/10.1371/journal.pone.0241320
Santos, D. C. M. (2009). Toxidez Aguda Do Zinco Em Lambaris Astyanax Aff. Bimaculatus. Mestrado Do Programa De Pósgraduação Em Biologia Animal, Universidade Federal De Viçosa, 125.
Silva, F. A. O. (2012). Por Uma Gestão Das Aguas Na Bacia Hidrográfica Do Rio Murucupi-Barcarena-PA. Dissertação De Mestrado Do Programa De Pós-Graduação Em Geografia, Universidade Federal Do Pará, 161.
Stankovic, S. Kalaba, P. Stankovi, A. R. (2014). Biota As Toxic Metal Indicators. Environ. Chem. Lett, 12, 63-84. https://doi.org/10.1007/s10311-013-0430-6
Usepa. (2000). Environmental Protection Agency. Methodology for deriving ambient water quality criteria for the protection of human health (2000): technical support document. Development of site-specific bioaccumulation factors, 185.
Viana, L. F. Cardoso, C. A. L. Lima-Junior, S. E. Súarez, Y. R. Florentino, A. C. (2020). Bioaccumulation of Metal in Liver Tissue of Fish in Response to Water Toxicity of the Araguari-Amazon River, Brazil. Environ. Monit. Assess, 192(781), 1-11. https://doi.org/10.1007/s10661-020-08696-2
Vu, C. T. Lin, C. Yeh, G. Villanueva, M. C. (2017). Bioaccumulation and Potential Sources of Heavy Metal Contamination in Fish Species in Taiwan : Assessment and Possible Human Health Implications. Environ. Sci. Pollut. Res, 24, 19422-19434. https://doi.org/10.1007/s11356-017-9590-4
Wassenaar, P. N. H. Verbruggen, E. M. J. Cieraad, E. Peijnenburg, W. J. G. M. Vijver, M. G. (2020). Variability in Fish Bioconcentration Factors: Influences of Study Design and Consequences for Regulation. Chemosphere, 239, 1-9. https://doi.org/10.1016/j.chemosphere.2019.124731
Wilson, R. W. Taylor, E. W. (1993). Differential Responses to Copper in Rainbow Trout, (Oncorhynchus Mykiss) Acclimated To Sea Water and Brackish Water. Journal of Comparative Physiology B, 163, 239-246. https://doi.org/10.1007/BF00261671
Yang, G. Sun, X. Song, Z. (2020). Trophic Level and Heavy Metal Pollution of Sardinella Albella in Liusha Bay, Beibu Gulf of The South China Sea. Marine Pollution Bulletin, 156, 1-8. https://doi.org/10.1016/j.marpolbul.2020.111204
Yi, Y. J. Zhang, S. H. (2012). The Relationships Between Fish Heavy Metal Concentrations and Fish Size in The Upper and Middle Reach of Yangtze River, Procedia Environmental Sciences, 13, 1699-1707. https://doi.org/10.1016/j.proenv.2012.01.163
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2022. All Rights Reserved.