
Original Article
INFLUENCE OF BACTERIOSPERMY ON THE LEVEL OF APOPTOSIS OF EJACULATED SPERMATOZOODS
INTRODUCTION
Since ejaculate is
a mixture of secretions obtained from the urogenital tract and male accessory
glands, seminal fluid culture reveals the presence of microbes in any part of
the seminal tract De Francesco et al. (2011). In recent years, increasing attention has
been paid to urogenital tract infections; many microorganisms may be involved
in the pathogenesis of diseases of the male reproductive system Shash et al.
(2023). Bacterial infiltration of the male
reproductive system triggers a local immune response, which is usually
accompanied by the release of cytokines and leukocytospermia
Frączek and Kurpisz (2015),Agarwal et al. (2018),Ventimiglia et al.(2020), which is often associated with a decrease
in male reproductive capacity. Finally, there is an opinion that bacterial
metabolism can alter the biochemical or physicochemical characteristics of
seminal plasma or the medium used for sperm processing, which may compromise
sperm survival both in vivo and in vitro Heidari Pebdeni et al. (2022). There is growing evidence that certain
bacterial species contribute to deterioration of sperm quality by directly
reducing sperm viability and motility, altering sperm morphology, and
indirectly affecting sperm quality through oxidative stress and immune or
autoimmune reactions Domes et
al. (2012). However, the most important mechanism
leading to the death of ejaculated sperm during urogenital tract
inflammation/infection is related to apoptosis.
Microorganisms
have been detected in semen Frączek and Kurpisz (2015) with varying effects on the reproductive
tract and sperm quality. However, no extensive studies have been conducted
linking the type of bacteriospermia and the level of
apoptosis in ejaculated sperm.
The aim of the
work: to conduct a comparative study of the influence of different types of bacteriospermia on the apoptosis of ejaculated human
spermatozoa.: to conduct a comparative study of the influence of different
types of bacteriospermia on the apoptosis of
ejaculated human spermatozoa.
Material and Methods
The study group
consisted of 20 healthy, fertile, normozoospermic
volunteers aged 20 to 35 years, recruited from the Astrakhan Center for Family Health and Reproduction, and 62 patients
with various types of bacteriospermia, treated at
urology hospitals in Astrakhan and Akhtubinsk. All
patients provided written consent to participate in the study. Semen samples
were obtained by masturbation after 3–5 days of sexual abstinence. After
liquefaction (30 minutes at room temperature), the samples were subjected to
routine semen analysis in accordance with recommendations published by the
World Health Organization Lutsky
et al. (2023). All samples were subjected to
microbiological analysis. Semen samples were plated on blood agar (BA) and
MacConkey agar (MCA) plates in the microbiology laboratory within 3 hours of
sample collection, according to WHO recommendations, followed by aerobic
incubation at 37°C for 24–48 hours. Samples with significant bacterial growth
(≥ 106 CFU/ml) were further tested to the species level using biochemical
identification tests. For Gram-positive bacteria: Gram stain, catalase, slide
coagulase, novobiocin, bacitracin, bile esculin, and optochin; for
Gram-negative bacteria: Gram stain, TSI agar (triple sugar iron), SIM test
(motility, indole, sulfur), Simmons citrate, urease,
and oxidase Koneman
et al. (1997). Semen samples with normal semen parameters,
no antisperm antibodies, and no signs of bacterial
infection (peroxidase-positive leukocytes <0.2 × 10 /ml and negative
bacterial culture) were selected as controls. Sperm from the collected semen
samples were separated from seminal plasma by centrifugation at 600 g for 8
min. The semen pellets were washed with warm phosphate-buffered saline (PBS),
pH 7.4, and adjusted to a final concentration of 4 × 10 sperm/ml PBS. Apoptosis
was analyzed by flow cytometry. The ANNEXIN V-FITC
APOPTOSIS DETECTION KIT I (BD Pharmingem™, USA) was
used to further confirm apoptosis. Sperm suspensions (2 × 10 6 /mL) were washed
once with PBS supplemented with Ca 2+ and then double-stained with annexin
V-FITC and propidium iodide (PI). After 15 min of incubation on ice in the
dark, the cells were diluted 1 × 1 with binding buffer consisting of 10 mM
HEPES, 140 mM NaCl, and 3.3 mM CaCl2. The apoptotic status in each group was
determined by flow cytometry on an Attune® NxT flow
cytometer according to the manufacturer's instructions, and the data were analyzed using FlowJo software.
Results and discussion
Among 82 semen
cultures (including controls), 47.56% had positive bacteriospermia,
of which Gram-negative bacteria were isolated with a significant predominance
(72.05% of all positive cultures). E. coli (14.63% of total cultures) was the
most frequently isolated bacterium, followed by K. pneumoniae (10.98%) and
Acinetobacter s (7.31%). Staphylococcus haemolyticus
(6.1%), Bacteroides ureolyticus (4.88%), and
Lactobacillus s (3.66%).
To quantify bacteriospermia-induced apoptosis in ejaculated
spermatozoa, flow cytometry analysis was performed after double labeling with Annexin V-FITC/PI. Sperm were isolated from
ejaculates with positive cultures for apoptosis assessment Table 1. Sperm from ejaculates with normal spermogram parameters and negative bacteriological results
were used as controls. Using multiparameter analysis and simultaneous cell
staining with nucleic acid dyes that do not penetrate living cells, such as
propidium iodide (PI), allows differentiation between cells in the early phase
of apoptosis (AnV+PI-), late apoptosis (AnV+PI+), and dead cells (AnV-PI+).
The ability to simultaneously assess membrane marker expression and AnV staining is highly valuable, allowing characterization
of the apoptotic cell population.
The cell profile
dot plots shown were obtained in 1 of 5 independent experiments that yielded
similar results. Quadrant Q1 reflects necrotic cells (AnV-PI+),
quadrant Q2 reflects late apoptosis (AnV+PI+),
quadrant Q3 reflects early apoptosis (AnV+PI-), and
quadrant Q4 reflects the percentage of viable cells (AnV-PI-).
As shown in Fig. 1, the proportion of apoptotic ejaculated spermatozoa
significantly increased overall in bacteriospermia. Table 1 was compiled based on the dot graphs, which
clearly reflects the dynamics of changes in the level of apoptotic spermatozoa.
Figure 1
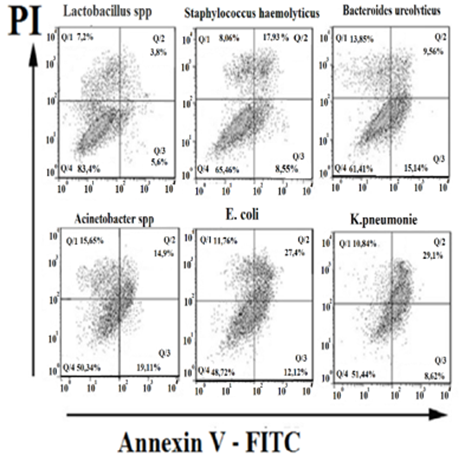
|
Picture 1 Flow
Cytofluorimetry of Apoptosis Induction in Ejaculated Sperm Depending on the
Type and Type of Microorganisms During Bacteriospermia.
Scatter Plots After Double Labeling with Annexin
V‑FITC and PI. The X-Axis Represents FITC Staining and the Y-Axis
Represents PI (Propidium Iodide) Staining. |
Externalization of
phosphatidylserine or early apoptosis in the case of Lactobacillus spp does not differ significantly from the control group,
but overall, the total apoptosis caused by Lactobacillus spp
is twice as high as the control Table 2. It has been suggested that lactobacilli
induce apoptosis due to the production of hydrogen peroxide, which causes
non-selective apoptosis Krüger
and Bauer (2017). A comparison of the early and late
apoptosis rates for various pathogens causing bacteriospermia
is also noteworthy. While Staphylococcus haemolyticus
and K. pneumoniae induce minor early apoptosis, late apoptosis under the
influence of these bacteria is much more pronounced Table 2. In the case of staphylococcal infection,
the main proapoptotic factors are numerous toxins characteristic of
staphylococci, including Staphylococcus haemolyticus.
Some toxins cause membrane damage and externalization of phosphatidylserine,
others activate caspases and DNA degradation, which is manifested in a positive
reaction to annexin and a positive reaction to propidium iodide (Ann+PI+), called late apoptosis Zhang et
al. (2017). K. pneumoniae exhibits high adhesive
capacity to the surfaces of various cells, thanks to P-glycoprotein and causes Ann+PI+ apoptosis, and also promotes transcriptional
expression of pro-inflammatory genes IL-6, IL-8, IL-1β and tumor necrosis factor (TNF)-α, as well as the
production of IL-8, IL-1β and TNF-α, which in turn are also
pro-apoptotic factors Cheng et
al. (2020).
Table 1
|
Table 1 Bacteriospermia.
Types and Types of Pathogens |
||||
|
pathogen |
Type |
Number
of positive cultures |
% From
the total number of crops |
% From
the number of positive cultures |
|
E. coli |
gram
negative |
12 |
14,63% |
30,77% |
|
K.
pneumoniae |
gram
negative |
9 |
10,98% |
23,08% |
|
Acinetobacter s |
gram
negative |
6 |
7,31% |
15,38% |
|
Staphylococcus
haemolyticus |
gram-positive |
5 |
6,1% |
12,82% |
|
Bacteroides
ureolyticus |
gram
negative |
4 |
4,88% |
10,26% |
|
Lactobacillus
s |
gram-positive |
3 |
3,66% |
7,69% |
Table 2
|
Table 2 Changes in the Level of Apoptosis of
Ejaculated Spermatozoa Depending on the Type and Kind of Microorganisms in Bacteriospermia (in the Table, all Reliabilities are
Calculated in Relation to the Control) |
||||||
|
type
of bacteria |
Аnn+PI- Early
apoptosis |
р |
Аnn+PI+ Late
apoptosis |
р |
Total Apoptosis |
р |
|
control |
3,8±0.72 |
- |
1,9±0,21 |
- |
4,7±0,61 |
- |
|
Lactobacillus
s |
5,6±0,38 |
0.0627 |
3,8±0,31 |
0.0267 |
9,4±0,35 |
0.00023 |
|
Staphylococcus
haemolyticus |
8,55±0,35 |
0.0008 |
17,93±0,35 |
0.000027 |
26,48±0,62 |
0.000001 |
|
Bacteroides
ureolyticus |
15.14±0,32 |
0.00002 |
9,56±2,75 |
0.0274 |
24,74±1,07 |
0.000001 |
|
Acinetobacter spp |
19,11±0,86 |
0.00003 |
14,9±1,9 |
0.000253 |
34,01±3,7 |
0.000106 |
|
E. coli |
12,12±0,84 |
0.0003 |
27,4±1,8 |
0.000002 |
39,52±2,56 |
0.000001 |
|
K.
pneumoniae |
8,62±0,8 |
0.00288 |
29,1±2,85 |
0.00003 |
37,72±3,83 |
0.000061 |
Gram-negative
bacteria (e.g., Escherichia coli) contain negatively charged molecules such as
phosphatidylglycerol and phosphates in their cell membranes Matsumoto (2001), whereas healthy mammalian cells mainly
contain phospholipids with a neutral charge, and bacterial lipids provoke the
externalization of phosphatidylserine, initiating the signaling
phase of apoptosis Boon and Smith (2002). Escherichia coli, a facultative anaerobe,
is a major cause of urinary tract infections Beebout
et al. (2022), and according to our data, bacteriospermia caused by Escherichia coli has the greatest
pro-apoptotic effect on ejaculated spermatozoa. Studies have shown that in
mouse cells infected with Escherichia coli (E. coli), there was an increase in
the content of IL-1β, IL-6, IL-8, TNF-α, leptin and resistin, and an increase in the levels of apoptotic
proteins (caspase-3, caspase-9 and bax/bcl-2) Guo et al. (2021).
Flow cytometry
also allows us to assess the percentage of necrotic AnV-PI+
cells, which is particularly important for assessing the viability of
ejaculated sperm. According to our data, the percentage of necrotic cells among
sperm isolated from semen containing Gram-positive bacteria averaged 7.9±0.62%,
while the percentage of necrotic cells in sperm isolated from semen containing
Gram-negative bacteria averaged 13.025±0.84%, which is 64% higher than in
Gram-positive bacteriospermia.
Conclusion
Thus, it is shown that semen contains unique
microbial profiles that appear to be characteristic of a certain subpopulation
of men. E. coli was the most frequently isolated bacterium, followed by K.
pneumoniae, Staphylococcus haemolyticus, Bacteroides ureolyticus, Lactobacillus s These observations are
consistent with previous studies Farahani
et al. (2021). Many of the bacterial species identified in
this study have a significant impact on the development of sperm apoptosis.
These are primarily gram-negative bacteria E. coli and K. pneumoniae. Many
types of bacteriospermia are characterized by a
decrease in the functional characteristics of sperm and, as a result,
subfertility and infertility Heidari Pebdeni et al. (2022). It can be speculated that microorganisms
can influence the environment in which sperm mature, thereby influencing their
physiology, in other words, inducing sperm apoptosis. Apoptosis induced by the
sperm microbiota may be one of the mechanisms underlying male infertility. The
development of new biomarkers for male infertility is crucial for improving the
diagnosis and prognosis of this disease
ACKNOWLEDGMENTS
None.
REFERENCES
Agarwal, A., Rana, M., Qiu, E., AlBunni, H., Bui, A. D., and Henkel, R. (2018). Role of Oxidative Stress, Infection and Inflammation in Male Infertility. Andrologia, 50(11), e13126. https://doi.org/10.1111/and.13126
Beebout, C. J., Robertson, G. L., Reinfeld, B. I., Blee, A. M., Morales, G. H., Brannon, J. R., and Chazin, W. J. (2022). Uropathogenic Escherichia Coli subverts Mitochondrial Metabolism to Enable Intracellular Bacterial Pathogenesis in Urinary Tract Infection. Nature Microbiology, 7(9), 1348–1360. https://doi.org/10.1038/s41564-022-01205-w
Boon, J. M., and Smith, B. D. (2002). Chemical Control of Phospholipid Distribution Across Bilayer Membranes. Medical Research Reviews, 22(3), 251–281. Https://Doi.Org/10.1002/Med.10009
Cheng, J., Zhang, J., Han, B., Barkema, H. W., Cobo, E. R., Kastelic, J. P., and Zhou, M. (2020). Klebsiella Pneumoniae Isolated from Bovine Mastitis is Cytopathogenic for Bovine Mammary Epithelial Cells. Journal of Dairy Science, 103(4), 3493–3504. https://doi.org/10.3168/jds.2019-17458
De Francesco, M. A., Negrini, R., Ravizzola, G., Galli, P., and Manca, N. (2011). Bacterial Species Present in the Lower Male Genital Tract: A Five-Year Retrospective Study. European Journal of Contraception and Reproductive Health Care, 16(1), 47–53. https://doi.org/10.3109/13625187.2010.533219
Domes, T., Lo, K. C., Grober, E. D., Mullen, J. B., Mazzulli, T., and Jarvi, K. (2012). The Incidence and Effect of Bacteriospermia and Elevated Seminal Leukocytes on Semen Parameters. Fertility and Sterility, 97(5), 1050–1055. https://doi.org/10.1016/j.fertnstert.2012.01.124
Farahani, L., Tharakan, T., Yap, T., Ramsay, J. W., Jayasena, C. N., and Minhas, S. (2021). The Semen Microbiome and its Impact on Sperm Function and Male Fertility: A Systematic Review and Meta-Analysis. Andrology, 9(1), 115–144. https://doi.org/10.1111/andr.12886
Frączek, M., and Kurpisz, M. (2015). Mechanisms of the Harmful Effects of Bacterial Semen Infection on Ejaculated Human Spermatozoa: Potential Inflammatory Markers in Semen. Folia Histochemica et Cytobiologica, 53(3), 201–217. https://doi.org/10.5603/FHC.A2015.0019
Frączek, M., Hryhorowicz, M., and Gill, K. (2016). The Effect of Bacteriospermia and Leukocytospermia on Conventional and Nonconventional Semen Parameters in Healthy Young Normozoospermic Males. Journal of Reproductive Immunology, 118, 18–27. https://doi.org/10.1016/j.jri.2016.08.006
Guo, H., Zuo, Z., Wang, F., Gao, C., Chen, K., Fang, J., Cui, H., Ouyang, P., Geng, Y., Chen, Z., Huang, C., Zhu, Y., and Deng, H. (2021). Attenuated Cardiac Oxidative Stress, Inflammation and Apoptosis in Obese Mice with Nonfatal Infection of Escherichia Coli. Ecotoxicology and Environmental Safety, 225, 112760. https://doi.org/10.1016/j.ecoenv.2021.112760
Heidari Pebdeni, P., Saffari, F., Mirshekari, T. R., Ashourzadeh, S., Taheri Soodejani, M., and Ahmadrajabi, R. (2022). Bacteriospermia and its Association with Seminal Fluid Parameters and Infertility in Infertile Men, Kerman, Iran: A Cross-Sectional Study. International Journal of Reproductive BioMedicine, 20(3), 202–212. https://doi.org/10.18502/ijrm.v20i3.10712
Koneman, E. W., Allen, S. D., Janda, W. M., Schreckenberger, P. C., and Winn, W. C. (1997). Diagnostic Microbiology: The Nonfermentative Gram-Negative Bacilli ( 253–320). Lippincott-Raven.
Krüger, H., and Bauer, G. (2017). Lactobacilli Enhance Reactive Oxygen Species-Dependent Apoptosis-Inducing Signaling. Redox Biology, 11, 715–724. https://doi.org/10.1016/j.redox.2017.01.015
Lutsky, D. L., Nikolaev, A. A., and Lutskaya, A. M. (2023). Guide to the Study of Human Ejaculate (2nd rev. ed.). Astrakhan.
Matsumoto, K. (2001). Dispensable Nature of Phosphatidylglycerol in Escherichia coli: Dual Roles of Anionic Phospholipids. Molecular Microbiology, 39(6), 1427–1433. https://doi.org/10.1046/j.1365-2958.2001.02320.x
Shash, R. Y. M., Mohamed, G. A. A., Shebl, S. E., Shokr, M., and Soliman, S. A. (2023). The Impact of Bacteriospermia on Semen Parameters Among Infertile Egyptian Men: A Case–Control Study. American Journal of Men’s Health, 17(3), 15579883231181861. https://doi.org/10.1177/15579883231181861
Ventimiglia, E., Capogrosso, P., Boeri, L., Cazzaniga, W., Matloob, R., Pozzi, E., Chierigo, F., and Abbate, C. (2020). Leukocytospermia is Not an Informative Predictor of Positive Semen Culture in Infertile Men: Results from a Validation Study of Available Guidelines. Human Reproduction Open, 2020(3), hoaa039. https://doi.org/10.1093/hropen/hoaa039
Zhang, X., Hu, X., and Rao, X. (2017). Apoptosis Induced by Staphylococcus Aureus toxins. Microbiological Research, 205, 19–24. https://doi.org/10.1016/j.micres.2017.08.006
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2026. All Rights Reserved.