
Enhanced Adsorptive Removal of Hexavalent Chromium using Aliquat-336 Impregnated Chitosan–Graphene Oxide Beads: Isotherm and Kinetic Studies
M. D. Bansinge 1![]() , P. V. Tekade 1
, P. V. Tekade 1
1 Department
of Chemistry, Bajaj College of Science, Wardha- 442001, India
|
|
ABSTRACT |
||
|
Aliquat-336
impregnated chitosan-graphene oxide (CHGOAL) was developed and tested as
efficient adsorbents to facilitate the removal of hexavalent chromium
(Cr(VI)) from aqueous media. The composite was a hybrid of the extensive
surface area and oxygen-rich functional moieties present on graphene oxide
and the amino groups of chitosan and the quaternary ammonium sites of
Aliquat-336 which increased the adsorption performance. FTTIR
characterization was used to verify interactions between components and XRD was
used to show an amorphous structure with dispersed graphene oxide. The impact
of the pH, the dosage of the adsorbent, contact time, and the initial
concentration of Cr(VI) were analyzed by batch adsorption experiments. The
equilibrium adsorption capacity of the adsorbent as per the Langmuir model
was 191.36 mg g-1 and was at pH 3 which had the maximum removal. The
pseudo-second-order model was appropriate to fit kinetic data (R2=0.998)
demonstrating that chemisorption constituted the rate-limiting step. The
CHGOAL beads proved to have high removal efficiency, structural stability,
and also reusability which is a good indication of their great application in
industrial wastewater treatment and environmental remediation. |
|||
|
Received 10 August 2025 Accepted 08 September 2025 Published 31 October 2025 Corresponding Author Manoj
Bansinge, bansinge@gmail.com DOI 10.29121/granthaalayah.v13.i10.2025.6472 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2025 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Chitosan–Graphene Oxide Composite,
Aliquat-336, Cr (VI) Removal, Chemisorption Mechanism |
|||
1. INTRODUCTION
Heavy metals may cause water pollution, which is a serious environmental issue because it is harmful, resistant in the environment, and can be accumulated in the food chain Saravanan et al. (2024), Zhang et al. (2023), and Singh et al. (2024). The danger of chromium hexavalent (Cr(VI)) is particularly severe due to the fact that this form of this element is highly soluble, mobile, and carcinogenic Sharma et al. (2022). Other industries like electroplating, tanning of leather, textile processing and metal surface finishing are also significant in Cr(VI) contamination of water. Prolonged contact with Cr(VI) has been linked to severe health issues, such as respiratory dysfunctions, skins, and cancer, making it critical to consider the methods to adequately remediate it Wise et al. (2022).
Adsorption, among many other treatment methods has proven effective, an economically viable and sustainable remediation approach to remove heavy metals due to simplicity and high efficiency at mild conditions Ashraf et al. (2024). Adsorbents that are based on bio-polymers and especially chitosan are of great interest due to their biodegradability, biocompatibility and large availability Singh et al. (2024). Chitosan, a derivative of chitin deacetylated, has amino and hydroxyl functional groups that facilitate the binding of metal ions via deacetylation, ion exchange, and chelation, electrostatic attraction mechanisms Ashraf et al. (2024). However, mechanic strength, solubility in acidic environment, and the lack of adsorption ability of certain pollutants are the most common reasons that restrict the practical use of chitosan.
Chitosan, bound to its use in the adsorption process, has been combined with graphene oxide (GO), a nanomaterial with a high surface area, high mechanical strength, and high oxygen functional groups Zhang et al. (2016). The chitosan-GO hybrid formed has better stability, reusability, and adsorption capacity, which form additional binding points to the heavy metal ions. A synergistic effect of chitosan and GO leads to the creation of high-performance adsorbents to remove Cr(VI).
The selectivity and efficacy of adsorption using Cr(VI) has been optimized by studying the functionalization of chitosan-GO using quaternary ammonium salts. The ammonium-based extractant aliquat-336 (trioctylmethylammonium chloride) is one of the well-known ammonium-based extractants that is commonly used in solvent extraction methods because it shows a high affinity to anionic species, such as Cr(VI) oxyanions (e.g., Cr₂O₇²⁻ and HCrO₄⁻), among others Kabay et al. (2003) , Alonso et al. (1997) , and Salazar et al. (1992). Its use in water systems is, however, usually restricted in solubility. The introduction of the Aliquat-336 into the solid matrix like chitosan-GO offers a robust and efficient system of Cr(VI) removal in addition to the limitations of the conventional solvent extraction.
We have synthesized a new chitosan-GO composite in this study which is modified with ammonium-based extractant (Aliquat-336) in the removal of Cr(VI) in water. Chitosan-GO framework provides structure stability and increased adsorption capacity, whereas the inclusion of the Aliquat-336 enhances a robust electrostatic connection with the Cr( VI) oxyanions. The adsorption performance of the composite was properly analyzed with the essential parameters being two aspects: the pH, duration of contact, initial concentration of Cr(VI), and temperature considered. Moreover, kinetics of adsorption, isotherm, and thermodynamics were discussed to comprehend more on the Cr(VI) removal mechanisms.
The study will be useful in the development of effective and environmentally friendly adsorbent materials to removes Cr(VI). The use of the chitosan-GO composite that is ammonium-based extractant is a promising solution to the challenges or problems of heavy metal contamination in industrial wastewater, which offers a viable, inexpensive, and eco-friendly alternative to conventional water treatment procedures.
2. Experimental
2.1. Materials
The degree of deacetylation >95% low molecular weight chitosan was purchased in SRL (India). Graphite (325 mesh) was purchased at 99.8% purity (Alfa Aesar, USA). Aliquat-336 (methyltrioctylammonium chloride, molecular weight 404.16, purity above 90%) was obtained at HiMedia (India). Potassium dichromate (K2Cr2O7) was purchased at Merck (India). The rest of the reagents and chemicals employed throughout the experiments were of analytical grade and were used as received to avoid any form of purification.
2.2. Synthesis of Graphene Oxide (GO)
Graphene oxide was synthesized via an enhanced version of the Hummers method (Yu et al., 2016). In summary, 2 g of graphite powder was put in a 250 mL flask (50 mL concentrated H2SO4 in it) and stirred in an ice bath. KMnO4 (6 g) was gradually introduced into the mixture during a period of 30 min at a temperature that was kept at a temperature below 5 °C and then left to stir during the next 2 hours. The mixture was heated up to 35 °C, and stirred mixed during another 2 hours, then 100 mL of deionized water was slowly added, which increased the temperature to 98 °C. The suspension was stirred at 98 °C and 30 mL of 30% H2O2 was added after which it was changed to a bright yellow suspension. The suspension was centrifuged into 5% HCl and then deionized water repeated till the mixture showed a neutral pH. A stable and homogeneous GO suspension was obtained by ultrasonic re-dispersal of the resulting GO in DI water
2.3. Preparation of Chitosan–Graphene Oxide (CHGO) Beads
The ionic gelation was used to prepare chitosan-graphene oxide beads (Kong et al., 2021). First, 2g chitosan was dissolved by adding 100mL of 2% (v/v) acetic acid solution and stirring continuously until the solution became transparent. Individually, 0.5 g of the produced GO was dispersed in 100 mL of deionized water and subjected to sonication for 30 min. The chitosan solution was stirred and the GO suspension was gradually added to it, so that it was distributed evenly. The obtained mixture was transferred into a syringe and added to a 0.5 M NaOH solution stirred. Ionic gelation of beads happened immediately and the beads were left in NaOH bath to harden in 2 hours. The formed CHGO beads were then washed in deionized water until the bead had a neutral pH and then dried in ambient condition over 24 hours.
2.4. Impregnation of CHGO Beads with Aliquat-336 (CHGOAL)
CHGOAL impregnation of CHGO Beads with Aliquat-336.
Aliquat-336 was placed in ethanol in a bid to make it more dispersed in order to impregnate the CHGO beads. Namely, 2 g of Aliquat-336 was dissolved in 20 mL of ethanol in a glass beaker with flotation stirring, resulting in a transparent solution of ions in liquid. 1 g of pre-dried CHGO beads was then added to this solution and the mixture then stirredmechanically until 12 hours of interaction between the Aliquat-336 and the bead matrix. The beads were then filtrated and briefly washed with small amounts of ethanol after the impregnation to remove the unadsorbed Aliquat-336 on the bead surfaces. The CHGOAL beads were then air-dried at room temperature during 12 hours to ensure that all the remaining solvent was driven off before their further characterization and adsorption. The solvent-aided technique facilitates even dispensing of Aliquat-336 in the surface and inside pores of the CHGO beads, enhancing the efficiency of the functionalization process.
2.5. Batch adsorption studies
Experiments of batch adsorption were conducted to assess the removal of Cr(VI) by adsorbent CHGOAL. In every run, 100 mg of the composite beads was weighed and 50 mL of aqueous potassium dichromate solution with 50 to 300 mg L-1 of Cr(VI) was added. The adsorption tests were done at the constant temperature of 298K and a contact time of 50 minutes under constant agitation to achieve homogenization. After the adsorption cycle, the filtration of the beads was done, and the concentration of the remaining Cr(VI) in the filtrate was measured spectrophotometrically, using the 1, 5- diphenylcarbazide (DPC) solution at a wavelength of 540 nm. Experiments were performed in triplicates so that it would be reproducible. The quantity of Cr ( VI ) adsorbed on the composite beads and also the percentage removal was determined in relation to known adsorption formulae.
q_e= (C_0-C_e)/W x V (1)
In this equation, C0 represents the starting concentration of Cr(VI) in the solution (mg L-1), Ce denotes the concentration at equilibrium, V expresses the volume of the solution in litres, and W indicates the mass of CHGOAL beads in grams employed during the adsorption experiment.
2.6. Physico-chemical Profiling
It is also important to evaluate the physico-chemical characteristics of the CHGOAL beads to determine how well they are suited and how best to make use of them in the removal of Cr (VI) in aqueous solutions. In this work, a descriptive characterization of the developed adsorbent is presented, along with the analysis of functional groups, crystal structure, and adsorption capacity of the engineered adsorbent to hexavalent chromium. There are several sophisticated methods of analysis, structural and compositional analysis, which were undertaken. The functional groups formed in the composite beads were determined by measuring Fourier-transform infrared (FTIR) spectra in 500-4000 cm-1 with a Bruker ATR spectrometer. X-ray diffraction (XRD) was used to determine the crystallinity and phase composition using a Rigaku Miniflex 600 diffractometer. Combined, these analyses can give an in-depth understanding of the structure-property correlation on the performance of the adsorbent in the removal of Cr(VI).
3. Result AND discussion
3.1. FTIR
The systematic functional changes are observed in the series of chitosan-based adsorbent through FTIR analysis. The characteristic bands of pristine chitosan (CH) include 3741.06 cm-1 (O-H/N-H stretching), 1535.49 cm-1 (amide II, N-H bending), and 988.72 cm-1 (C-O stretching), which proves the polysaccharide character of chitosan with active amine bonds (C–O stretching), confirming its polysaccharide structure with reactive amine sites (B. Zhang et al., 2018). The O-H band in CHGO is shifted to 3266.96 cm-1 and the amide II and C-O bands are located at 1567.63 cm-1 and 1016.81 cm-1 and these changes confirm the presence of strong hydrogen bonding and the establishment of new ether/ester connections with graphene oxide. The integration of the quaternary ammonium ionic liquid is confirmed at 2920.54 cm-1 (C-H stretching) and 1376.37 cm-1 (C-N+ stretching) with fresh absorption peaks on Aliquat-336 impregnation (CHGOAL) (Ranjbari et al., 2019). The change of C-O bands to 1024.63 cm-1 with slight change to 1024.63 cm-1 shows the interaction of the ionic liquid with the polymer matrix Lin et al. (2022). The CHGOAL-Cr spectra indicate that post Cr(VI) adsorption has a C-O shift to 1017.29 cm-1 and fewer intensities of hydroxyl /amphetamine bands, indicating their involvement in metal coordination. The tenacity of quaternary ammonium vibrations confirms the presence of anion-exchange with Cr(VI) oxyanions. All these spectral changes establish the gradual functionalization and collaborative functions of hydroxyl, amine and quaternary ammonium groups to bind Cr(VI) through chelation and exchange of anions.
Figure 1
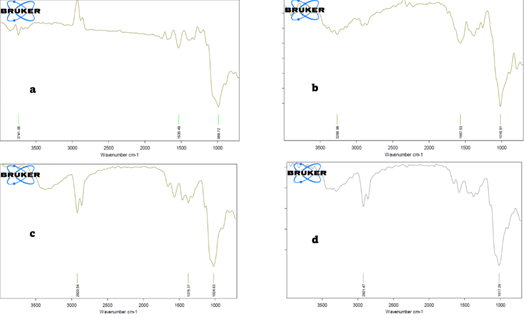
|
Figure 1 FTIR Spectra Of (A) Chitosan, (B) CHGO (C) CHGOAL & (D) After Adsorption of Cr (VI) |
3.2. XRD
The diffraction patterns obtained from X-ray analysis of the four samples i.e., (a) chitosan, (b) CHGO (chitosan-graphene oxide beads), (c) CHGOAL beads and (d) the composite following the adsorption of Cr(VI) reveal the crystal differences in crystallinity and structural arrangement during every step of modification and utilization. The XRD pattern of chitosan (a) indicates one broad peak at 2θ ≈ 20°, which is characteristic of the semi-crystalline structure of chitosan Balakrishnan et al. (2023). The peak becomes broader and smaller after incorporation of GO (b, CHGO), and that means the disorder increases. The further reduction in the intensity of the peak by aliquat-336 impregnation (c) depicts the increased amorphousness Lin et al. (2022). After adsorption of post Cr(VI) (d) the general trend remains, but with minor variations indicating that chromium is taken up without the development of new crystalline phases.
Figure 2
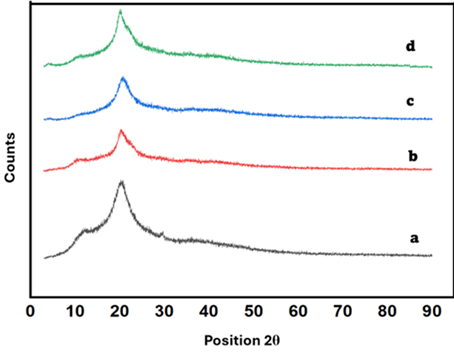
|
Figure 2 Stacked XRD Spectra Of (A) Chitosan, (B) CHGO (C) Aliquat-336 Impregnated CHGO & (D) After Adsorption of Cr (VI) |
3.3. Effect of pH
The pH represents a key parameter affecting the removal of Cr(VI) through adsorption because the surface charge of adsorbent and ionic speciation of chromium is influenced. The experiments were conducted by changing the pH of Cr(VI) solution (50 mL, 50mg L-1) by adding dilute HCl or NaOH until pH 2-9. As illustrated in Fig. 3a, the adsorption efficiency rose drastically across more reduced pH reaching a maximum removal at pH 3. Such an improvement in adsorption is related to the positive charge of the amino groups on the chitosan matrix and a negative charge of the chromate species (HCrO₄⁻, Cr₂O₇²⁻). At pH higher than 3, there was a slow decline in adsorption as competition by hydroxyl ions (OH-) started to increase and also because HCrO₄⁻ to CrO₄²⁻, which has lesser affinity to the positively charged surface, appeared. Therefore, the pH of 3 was chosen as the best condition to use in further experiments.
3.4. Effect of Adsorbent Amount
Adsorbent dosage effect was studied by adjusting the mass of the adsorbent to 25-150 mg, maintaining the concentration of initial Cr(VI) (50mg L-1) and the volume of solution (50mL) constant. Fig. 3b shows that the percent removal of Cr(VI) was proportional to the amount of adsorbent and was thus at its highest efficiency at 100 mg. This is as a result of increased availability of active binding sites to adsorption of Cr(VI). But on addition of above 100 mg, the removal efficiency was almost at a constant and this implies that the equilibrium was achieved and no further addition of adsorbent was found to have a significant effect on adsorption. This minimal decrease at the highest dosages can be explained by agglomeration of the particles decreasing the amount of effective surface area that is available to be adsorbed.
3.5. Effect of Contact Time
Influence of the contact time on the Cr(VI) adsorption was investigated by changing the contact time between 5 and 120 minutes under the same conditions (pH 3, 50 mg L-1 Cr(VI), 100 mg adsorbent). The findings in Fig. 3c indicate that at the early stages (0-40 min), the adsorption was very fast and this is due to high density of active sites on the adsorbent surface. The equilibrium was reached at 50 minutes with almost 100 percent adsorption. Further on, the adsorption rate was insignificant as binding sites were saturated. The trend indicates that the process is two-stage adsorption; initially, the adsorption is rapid on the surface, and then slower intraparticle diffusion takes place, which is representative of pseudo-second-order kinetics.
3.6. Effect of Initial Cr(VI) Concentration
The influence of initial concentration of Cr(VI) on adsorption capacity was examined by changing the concentration (10-300 mg L -1) and keeping a constant dosage of the adsorbent (100 mg) and volume (50 mL). Fig. 3d shows that the adsorption capacity (qe) was greater with an increase in the concentration of Cr(VI) due to the stronger force of mass transfer and higher velocity of Cr(VI) ions to adsorbent surface. Nevertheless, it was found that when the concentration was very high (>200 mg L-1), the percentage removal became lower, meaning that the adsorption sites were saturated. This effect indicates the limited number of active sites and the Langmuir isotherm model in which monolayer adsorption is confirmed on a homogeneous surface.
Figure 3
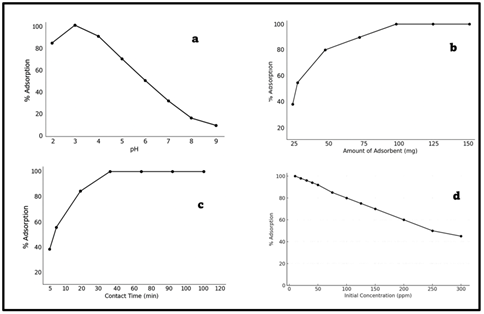
|
Figure 3 Effect Of (A) Ph, (B) Adsorbent Dose, (C) Contact Time, (D) Initial Cr(VI) Concentration on Adsorption Efficiency. |
3.7. Adsorption kinetics
The kinetics of Cr (VI) adsorption onto Aliquat-336 impregnated with chitosan-graphene oxide beads were studied under a starting concentration of 50 mg L-1, 100mgadsorbent and 50 ml solution at pH 3. The percentage of adsorption rose quickly in the initial 40 min after which the percentage reached 100 percent removal (after 50 min) thereafter no significant increase was observed suggesting the attainment of equilibrium. Pseudo-First-Order (PFO), Pseudo-Second-Order (PSO), Intraparticle Diffusion (IPD), and Elovich models were applied to analyze the kinetic data in order to explain the rate controlling mechanism of adsorption. The linear plots of each kinetic model have been provided in Fig. 5 and the estimated parameters have been summarized in Table 1 Kinetic Model Parameters for Cr (VI) Adsorption. It was observed that the PSO model gave the highest correlation to the experimental data (R2 = 0.998) indicating that the adsorption process is chemisorption kinetics based on the valence forces after the exchange of electrons between the Cr(VI) ions and the functional groups of the Aliquat-336 impregnated chitosan-graphene oxide composite. The experimentally measured adsorption capacity compared very closely with the predicted by the PSO model adsorption capacity (qe = 26.96 mg g-1) which further confirmed the applicability of this model. The value of rate constant (k2) was 0.00524 g mg-1 min-1. The PFO model revealed a less strong correlation coefficient (R2 = 0.723), which means that there was no capacity to explain the overall kinetic behaviour using only physical adsorption. The Elovich model had moderate fit (R2 = 0.922) that is characteristic of surface heterogeneity and changing adsorption site activation energies. The coefficients a ( 10.54 mg g-1 min-1 ) and b (0.203 g mg-1 ) indicate an initial rapid adsorption with the successive saturation of sites. The Intraparticle Diffusion (IPD) model showed a multi-linear plot, which proved that the process of adsorption proceeded in more than single steps. The initial linear part is associated to film diffusion and the second is the pore diffusion in the adsorbent structure. The value of C ( = 10.73 mg g-1) indicates that the rate is strongly dependent on the effects of the boundary-layer, and the values of R2 ( = 0.769) indicate that the diffusion rate is not only determined by intraparticle diffusion. On the whole, the kinetic study indicates that the kinetic process of adsorption of Cr(VI) ions onto Aliquat-336 impregnated chitosan-graphene oxide beads takes place majorly through chemisorption, with pseudo-second-order kinetic, where both the film and intraparticle diffusion processes play a role.
Figure 4
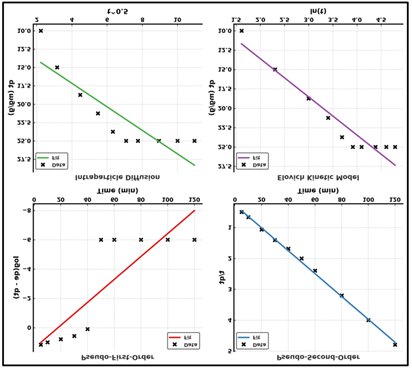
|
Figure 4 Kinetic Plots for Cr (VI) Adsorption onto Aliquat-336 Impregnated Chitosan–Graphene Oxide Composite: (A) Pseudo-First-Order, (B) Pseudo-Second-Order, (C) Intraparticle Diffusion, (D) Elovich Model. |
Table 1
|
Table 1 Kinetic Model Parameters for Cr (VI) Adsorption |
||
|
Kinetic
Model |
Parameters |
Calculated
Values |
|
Pseudo-First-Order Kinetics |
k1 (min-1) R2 |
0.18 0.723 |
|
Pseudo-Second
Order |
k2
(g mg-1min-1) R2 qe.
exp (mg g-1) qe.
cal (mg g-1) |
0.00524 0.998 25 26.96 |
|
Intraparticle
Diffusion |
kp
(mg g-1min-1/2) C
(Boundary Layer Constant) R2 |
1.604 10.73 0.769 |
|
Elovich
Model |
α
(mg g-1min-1) β
(g mg-1) R2 |
10.54 0.203 0.922 |
3.8. Adsorption isotherms
The optimized experimental conditions were studied on the equilibrium adsorption data of hexavalent chromium (Cr ( VI )) removal using Aliquat-336 impregnated Chitosan-Graphene oxide composite beads. The adsorption studies were conducted at pH 3, where 50 mL of Cr(VI) solutions of initial concentrations of 10 to 300 mg L-1 were used and the adsorbent dosage used was 100mg. Once the equilibrium was achieved, the filtrate was measured with DPC spectrophotometrically to measure the remaining Cr(VI) concentration. In order to characterize the properties of the equilibrium, the data were modelled with six frequently used isotherm models, which include Langmuir, Freundlich, Temkin, Dubinin-Radushkevich (D-R), Elovich and Redlich-Peterson (R-P). These models were linearized and used to estimate the isotherm constants and the correlation coefficients (R2), which were used as a measure of the goodness of fit. Fig 6, Of all the models that were tested, the Langmuir model was the most correlated (R2 = 0.9999), which showed that the adsorption of Cr(VI) is a monolayer process on a homogeneous surface. The experimental value was well following the calculated maximum adsorption capacity (qe = 191.37 mg g-1) and this showed that the model is reliable. The fact that the Freundlich constant (n = 2.75) was positive indicated good adsorption on a slightly heterogeneous surface, whereas the Temkin constant (B = 16.54 J mol-1) indicated an intermediate heat of adsorption which is characteristic of physisorption. An additional test was made on the Dubinin radushkevich mean free energy ( E = 4.52 kJ mol-1) which further established that the interaction between Cr( VI) ions and adsorbent surface was physical in nature. Redlich-Peterson model (β = 0.94) had the traits between Langmuir and Freundlich equations and the system was in Langmuir-type monolayer adsorption with minor heterogeneity of the surface. The Elovich isotherm also showed that it fit well (R2 = 0.958), which indicates the existence of multilayer tendencies of physical adsorption, at high solute concentrations. To conclude, the isotherm study indicates that the adsorption of Cr(VI) ions onto the Aliquat-336 impregnated Chitosan-Graphene oxide adsorbent is a monolayer, favourable and physisorption ruled out isotherm, and the process follows primarily the Langmuir isotherm model.
Figure 5
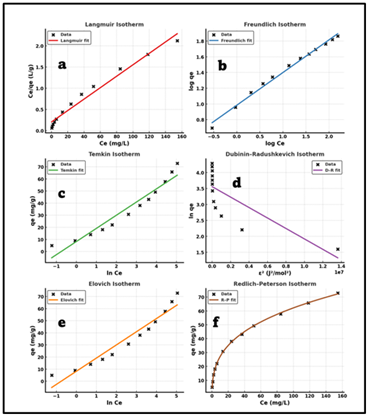
|
Figure 5 (A) Langmuir Isotherm, (B) Freundlich Isotherm, (C) Temkin Isotherm, (D) D-R Isotherm. (E) Elovich Isotherm, (F) R-P Isotherm. |
Table 2
|
Table 2 Adsorption Parameters |
||
|
Isotherm
Model |
Parameters |
Calculated
Values |
|
Langmuir
Isotherm |
qm (mgg-1) KL (Lmg-1) R2 |
191.37 0.0299 0.999 |
|
Freundlich
Isotherm |
KF (mgg-1) n R2 |
4.48 2.75 0.972 |
|
Temkin
Isotherm |
A (Lg-1) b
(Jmol-1) R2 |
2.67 16.543 0.951 |
|
D-R
Isotherm |
qm (mgg-1) β
(mol²J-²) E
(kJ mol-1) R2 |
182.6 2.45×10−9
4.52 0.932 |
|
R-P Isotherm |
KR
(Lg-1) aR β R2 |
1.876 0.0124 0.94 0.998 |
|
Elovich Isotherm |
α
(mgg-1min-1) β
(gmg-1) R2 |
1.78×10−2 0.038 0.958 |
3.9. Adsorption Mechanism Discussion
The adsorption process of the hexavalent chromium [Cr(VI)] onto Aliquat-336 impregnated beads of chitosan-graphene oxide (CS-GO-Aliquat) can be explained by a combination of synergistic forces that work together in the solid-solution interface, which includes the electrostatic attraction, ion exchange, and complexation. When the pH is optimized at 3, the amino (-NH2) groups of chitosan can be protonated into -NH3+ allowing a strong electrostatic attraction between the chitosan and the negatively charged chromate species (HCrO₄⁻ and Cr₂O₇²⁻) Shekhawat et al. (2015). Graphene oxide adds a significant surface area and oxygenated functional groups (-COOH, -OH, and -C=O) that increase the dispersion of chitosan and offers more binding sites that hydrogen bond and form surface complexes Samuel et al. (2019). This two-fold capacity of chitosan-Aliquat interaction becomes an electrostatic interaction with Aliquat and an ion-exchange with Aliquat providing a cooperating adsorption environment that facilitates fast diffusion and an increased site accessibility.
3.10. Comparative Study
The results of the comparative analysis of the adsorption capacities Table 3 Comparison of Cr (VI) Adsorption Capacities of Chitosan-Based Adsorbents indicate high performance of the Aliquat -336 impregnated chitosan- graphene oxide (CHGO) composite. Pristine chitosan (90.76 mg g-1) and chitosan/GO (67.8 mg g-1) had lesser capacities, and the chitosan-Aliquat-336 composite was 110 mg g-1 as reported in the study by Lin et al. (2022). By comparison, the current CHGO composite had an adsorption capacity of 191.36 mg g-1, which is due to the synergistic action of the amino groups of chitosan, the high surface area of the graphene oxide, and the quaternary ammonium sites of Aliquat-336. This mixture has increased the attraction of electrostatic forces, ion exchange, and surface complexation leading to quick and efficient removal of Cr(VI), which proves that CHGO is a good candidate in the treatment of industrial wastewater.
Table 3
|
Table 3 Comparison of Cr (VI) Adsorption Capacities of Chitosan-Based Adsorbents |
||
|
Sorbent Material |
Adsorption Capacity (mg g-1) |
Reference |
|
Chitosan
beads |
90.76 |
(Xu et al.,
2024) |
|
Chitosan/GO
Composite |
67.8 |
(Wu et al.,
2017) |
|
Chitosan-Aliquat-336
Composite |
110 |
(Lin et
al., 2022) |
|
Aliquat-336 impregnated CHGO |
191.36 |
Present
study |
4. Conclusion
Aliquat-336-impregnated chitosan-graphene oxide beads have been prepared effectively as a bio-based hybrid adsorbent, which can be used in the effective removal of Cr(VI) in aqueous solutions. The adsorption was highly pH-dependent with the best removal at pH 3 that was due to electrostatic attraction between the protonated amino groups and the anionic chromate species. The Langmuir model is the one which fits equilibrium data (R2 = 0.999), which suggests that the monolayer is adsorbed with a top capacity of 191.36 mg g-1. A pseudo-second-order model (R2 = 0.998) was used to conduct kinetic studies that indicated that the process was controlled by chemisorption. Combination of chitosan, graphene oxide and Aliquat-336 was synergistic, which increased adsorption and structural stability. In general, the composite shows great potential and as an environmental-friendly system in industrial effluent treatment and chromium-contaminated water detoxification, which can be used as long as the environment is sustainably remedied.
AUTHOR CONTRIBUTIONS
M. D. Bansinge: Responsible for data curation, formal analysis, and drafting the original manuscript. P. V. Tekade: Provided supervision, managed software-related tasks, and ensured the availability of resources and Contributed to reviewing and editing the manuscript
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
The authors sincerely appreciate the laboratory facilities provided by the Bajaj College of Science, Wardha.
REFERENCES
Alonso, A. I., Galan, B., Irabien, A., and Ortiz, I. (1997). Separation of Cr(VI) with Aliquat 336: Chemical Equilibrium Modeling. Separation Science and Technology, 32(9), 1543–1555. https://doi.org/10.1080/01496399708004065
Ashraf, A., Dutta, J., Farooq, A., Rafatullah, M., Pal, K., and Kyzas, G. Z. (2024). Chitosan-Based Materials for Heavy Metal Adsorption: Recent Advancements, Challenges and Limitations. Journal of Molecular Structure, 1309, 138225. https://doi.org/10.1016/j.molstruc.2024.138225
Balakrishnan, A., Appunni, S., Chinthala, M., Jacob, M. M., Vo, D.-V. N., Reddy, S. S., and Kunnel, E. S. (2023). Chitosan-Based Beads as Sustainable Adsorbents for Wastewater Remediation: A Review. Environmental Chemistry Letters, 21(3), 1881–1905. https://doi.org/10.1007/s10311-023-01563-9
Kabay, N., Arda, M., Saha, B., and Streat, M. (2003). Removal of Cr(VI) by Solvent Impregnated Resins (SIR) Containing Aliquat 336. Reactive and Functional Polymers, 54(1), 103–115. https://doi.org/10.1016/S1381-5148(02)00186-4
Kong, D., He, L., Li, H., Zhang, F., and Song, Z. (2021). Preparation and Characterization of Graphene Oxide/Chitosan Composite Aerogel with High Adsorption Performance for Cr(VI) by a New Crosslinking Route. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 625, 126832. https://doi.org/10.1016/j.colsurfa.2021.126832
Lin, X., He, X., Lei, L., Zhao, Y., Cui, L., and Wu, G. (2022). Development of Ionic Liquid Filled Chitosan Capsules to Remove Cr(VI) from Acidic Solution: Adsorption Properties and Mechanism. Journal of Environmental Chemical Engineering, 10(4), 108081. https://doi.org/10.1016/j.jece.2022.108081
Ranjbari, S., Tanhaei, B., Ayati, A., and Sillanpää, M. (2019). Novel Aliquat 336 Impregnated Chitosan Beads for the Adsorptive Removal of Anionic Azo Dyes. International Journal of Biological Macromolecules, 125, 989–998. https://doi.org/10.1016/j.ijbiomac.2018.12.139
Salazar, E., Ortiz, M. I., Urtiaga, A. M., and Irabien, J. A. (1992). Equilibrium and Kinetics of Chromium(VI) Extraction with Aliquat 336. Industrial & Engineering Chemistry Research, 31(6), 1516–1522. https://doi.org/10.1021/ie00006a014
Samuel, M. S., Bhattacharya, J., Raj, S., Santhanam, N., Singh, H., and Pradeep Singh, N. D. (2019). Efficient Removal of Chromium(VI) from Aqueous Solution Using Chitosan Grafted Graphene Oxide (CS–GO) Nanocomposite. International Journal of Biological Macromolecules, 121, 285–292. https://doi.org/10.1016/j.ijbiomac.2018.09.170
Saravanan, P., Saravanan, V., Rajeshkannan, R., Arnica, G., Rajasimman, M., Baskar, G., and Pugazhendhi, A. (2024). Comprehensive Review on Toxic Heavy Metals in the Aquatic System: Sources, Identification, Treatment Strategies, and Health Risk Assessment. Environmental Research, 258, 119440. https://doi.org/10.1016/j.envres.2024.119440
Sharma, P., Singh, S. P., Parakh, S. K., and Tong, Y. W. (2022). Health Hazards of Hexavalent Chromium (Cr(VI)) and its Microbial Reduction. Bioengineered, 13(3), 4923–4938. https://doi.org/10.1080/21655979.2022.2037273
Shekhawat, A., Kahu, S., Saravanan, D., and Jugade, R. (2015). Synergistic Behaviour of Ionic Liquid Impregnated Sulphate-Crosslinked Chitosan Towards Adsorption of Cr(VI). International Journal of Biological Macromolecules, 80, 615–626. https://doi.org/10.1016/j.ijbiomac.2015.07.035
Singh, V., Ahmed, G., Vedika, S., Kumar, P., Chaturvedi, S. K., Rai, S. N., Vamanu, E., and Kumar, A. (2024). Toxic Heavy Metal Ions Contamination in Water and their Sustainable Reduction by Eco-Friendly Methods: Isotherms, Thermodynamics and Kinetics Study. Scientific Reports, 14(1), 7595. https://doi.org/10.1038/s41598-024-58061-3
Wise, J. P., Young, J. L., Cai, J., and Cai, L. (2022). Current Understanding of Hexavalent Chromium [Cr(VI)] Neurotoxicity and New Perspectives. Environment International, 158, 106877. https://doi.org/10.1016/j.envint.2021.106877
Wu, L., Qin, Z., Yu, F., and Ma, J. (2017). Graphene Oxide Cross-Linked Chitosan Nanocomposite Adsorbents for the Removal of Cr(VI) from Aqueous Environments. Desalination and Water Treatment, 72, 300–307. https://doi.org/10.5004/dwt.2017.20648
Xu, S., Wang, Y., Wei, M., Li, Y., Qi, P., Li, R., and Xing, Y. (2024). Removal of Cr(VI) by Crosslinked Chitosan Adsorbent Prepared using Simulated Copper-Containing Wastewater. International Journal of Biological Macromolecules, 282, 137301. https://doi.org/10.1016/j.ijbiomac.2024.137301
Yu, H., Zha, B., Chaoke, B., Li, R., and Xing, R. (2016). High-Efficient Synthesis of Graphene Oxide Based on Improved Hummers Method. Scientific Reports, 6, 36143. https://doi.org/10.1038/srep36143
Zhang, B., Hu, R., Sun, D., Wu, T., and Li, Y. (2018). Fabrication of Chitosan/Magnetite-Graphene Oxide Composites as a Novel Bioadsorbent for Adsorption and Detoxification of Cr(VI) from aqueous solution. Scientific Reports, 8(1), 15397. https://doi.org/10.1038/s41598-018-33925-7
Zhang, L., Luo, H., Liu, P., Fang, W., and Geng, J. (2016). A Novel Modified Graphene Oxide/Chitosan Composite used as an Adsorbent for Cr(VI) in Aqueous Solutions. International Journal of Biological Macromolecules, 87, 586–596. https://doi.org/10.1016/j.ijbiomac.2016.03.027
Zhang, P., Yang, M., Lan, J., Huang, Y., Zhang, J., Huang, S., Yang, Y., and Ru, J. (2023). Water Quality Degradation Due to Heavy Metal Contamination: Health Impacts and Eco-Friendly Approaches for Heavy Metal Remediation. Toxics, 11(10), 828. https://doi.org/10.3390/toxics11100828
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2025. All Rights Reserved.