
Integrative Omics Approaches in Unraveling Plant Stress Mechanisms
Dr. Ragini Sikarwar 1
1 Assistant
Professor & HOD, Department of Botany Government Homescience
PG Lead College, Narmadapuram (MP), India
|
|
ABSTRACT |
||
|
Plants encounter a wide array of abiotic and biotic stresses, including drought, salinity, extreme temperatures, pathogens, and heavy metals, which severely limit agricultural productivity. Understanding stress responses at the molecular level is crucial for developing resilient crops. In recent years, omics technologies—genomics, transcriptomics, proteomics, metabolomics, and phenomics— have emerged as powerful tools for dissecting the complex regulatory networks governing plant stress adaptation. This article reviews advances in integrative omics approaches that provide holistic insights into plant stress tolerance mechanisms. Genomics has facilitated the identification of stress-responsive genes, while transcriptomics reveals dynamic gene expression under stress. Proteomics and metabolomics provide information on protein modifications and metabolic fluxes, respectively, and phenomics bridges molecular data with physiological responses. Integrating multi-omics datasets with computational modeling and systems biology enables the construction of predictive networks for stress resilience. Case studies
on Arabidopsis thaliana, rice (Oryza sativa), wheat (Triticum aestivum), and
soybean (Glycine max) demonstrate the power of omics integration in
identifying biomarkers, regulatory hubs, and candidate genes for crop
improvement. Challenges remain in data standardization, big data analysis,
and translating omics insights into field applications. Nevertheless,
integrative omics promises a transformative path toward sustainable
agriculture in the era of climate change. |
|||
|
Received 10 June 2025 Accepted 18 July 2025 Published 31 August 2025 DOI 10.29121/granthaalayah.v13.i8.2025.6415 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2025 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Plant Stress, Omics Integration,
Genomics, Transcriptomics, Proteomics, Metabolomics, Systems Biology
|
|||
1. INTRODUCTION
Plant growth and productivity are continually challenged by environmental stresses, both abiotic (drought, salinity, cold, heat, heavy metals) and biotic (pathogens, herbivores). These stresses lead to significant yield losses worldwide, threatening food security. For instance, drought and salinity alone are estimated to reduce crop productivity by more than 50% globally Boyer (1982), Zandalinas et al. (2021).
Traditional breeding approaches, while successful in improving crop traits, have limitations due to the complexity and polygenic nature of stress tolerance. With the advent of high-throughput sequencing and omics platforms, researchers now have unprecedented opportunities to unravel the intricate molecular responses of plants under stress. Integrative omics approaches combine multiple datasets—DNA, RNA, protein, metabolite, and phenotype information—to provide a comprehensive understanding of stress adaptation mechanisms.
This article elaborates on the principles, applications, advantages, and limitations of omics-based strategies in plant stress biology, with emphasis on their role in crop improvement.
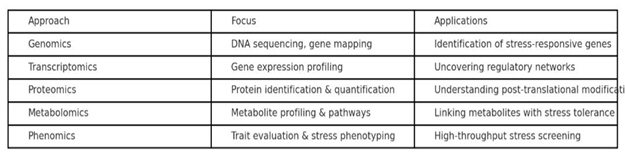
2. Omics Approaches in Plant Stress Research
1) Genomics
Genomics lays the foundation by identifying stress-responsive genes and QTLs.
Whole-genome sequencing of major crops like rice, wheat, and maize has provided gene catalogs for stress adaptation International Rice Genome Sequencing Project. (2005).
Genome-wide association studies (GWAS) link natural genetic variation with stress tolerance traits.
CRISPR/Cas9 and TALENs further enable targeted manipulation of stress-related loci.
Case Example: Genomic studies in rice identified Sub1A, a major gene conferring submergence tolerance, now widely used in breeding submergence-tolerant rice varieties Xu et al. (2006).
2) Transcriptomics
Transcriptome analysis reveals differential gene expression under stress.
RNA-Seq provides high-resolution profiling of stress-induced transcripts.
Non-coding RNAs (miRNAs, lncRNAs) regulate stress responses by modulating gene expression.
Case Example: Transcriptome profiling of wheat under drought stress identified transcription factors (DREB, NAC, MYB) that regulate stress-responsive genes Gupta et al. (2020).
3) Proteomics
Proteomics helps in understanding post-translational modifications and protein–protein interactions during stress.
2D gel electrophoresis and mass spectrometry detect protein abundance changes.
Phosphoproteomics identifies signaling cascades activated under stress.
Case Example: Proteome analysis in soybean under salinity stress revealed enhanced abundance of antioxidant enzymes, reflecting stress mitigation strategies Ma et al. (2016).
4) Metabolomics
Metabolomics captures changes in small molecules that reflect the plant’s physiological state.
Gas chromatography–mass spectrometry (GC–MS) and LC–MS are widely used.
Stress alters metabolites such as proline, sugars, and secondary metabolites that act as osmoprotectants and antioxidants.
Case Example: Metabolomic profiling of maize under drought stress revealed accumulation of proline and raffinose, key osmoprotectants that aid stress tolerance Obata et al. (2015).
5) Phenomics
Phenomics bridges molecular information with whole-plant traits.
High-throughput imaging and sensors measure plant growth, morphology, and photosynthesis.
Integration with genomics and metabolomics aids in identifying quantitative stress-response traits.
2.1. Integrative Omics and Systems Biology
The real power of omics lies in integration
Multi-omics integration (e.g., transcriptomics + metabolomics) reveals how gene expression translates into metabolic changes.
Network biology constructs regulatory maps linking transcription factors, enzymes, and metabolites.
Machine learning and AI models predict plant responses under stress and suggest breeding targets.
Case Example: Integrated transcriptome and metabolome analysis in Arabidopsis under heat stress identified HSFA1 transcription factor as a master regulator of stress tolerance Li et al. (2020).
3. Results and Discussion
Case Studies of Omics in Plant Stress:
1) Rice (Oryza sativa): Integrative genomics and transcriptomics identified drought-responsive genes, enabling development of drought-tolerant varieties.
2) Wheat (Triticum aestivum): Multi-omics studies revealed the role of ABA signaling pathways in salinity tolerance.
3) Soybean (Glycine max): Proteome and metabolome integration uncovered antioxidant pathways crucial for oxidative stress resistance.
4) Arabidopsis thaliana: Served as a model for heat and cold stress, aiding in the identification of universal stress regulators.
3.1. Advantages
· Provides holistic insights into stress biology.
· Identifies biomarkers and regulatory hubs for crop improvement.
· Accelerates translational research in breeding programs.
3.2. Limitations
· High cost and technological barriers.
· Data standardization and reproducibility issues.
· Requirement for advanced computational infrastructure.
4. Conclusion
Integrative omics has emerged as a transformative tool for understanding and improving plant stress tolerance. By combining genomic, transcriptomic, proteomic, metabolomic, and phenomic data, researchers can construct a comprehensive systems-level view of stress responses. While challenges persist in data integration, computational modeling, and field validation, the synergy of omics with genome editing, AI, and precision agriculture holds great promise. These strategies will not only enhance crop productivity but also contribute to global food and nutritional security under the looming threat of climate change.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
Boyer, J. S. (1982). Plant Productivity and Environment. Science, 218(4571), 443-448.
Gupta, P., Redden, R., Sharma, R., & Varshney, R. K. (2020). Advances in Understanding Drought Tolerance in Wheat. Theoretical and Applied Genetics, 133(6), 1601-1620. https://doi.org/10.1007/s00122-020-03583-3
International Rice Genome Sequencing Project. (2005). The Map-Based Sequence of the Rice Genome. Nature, 436(7052), 793-800. https://doi.org/10.1038/nature03895
Li, H., Chang, J., Chen, H., Wang, Z., & Gu, X. (2020). Integrative Analysis of Heat Stress Responses in Arabidopsis. Plant Physiology, 183(4), 1660-1677.
Ma, H., Zhao, J., & Meng, X. (2016). Proteomic Analysis of Soybean Response to Salinity Stress. Journal of Proteomics, 143, 73-87.
Obata, T., Witt, S., Lisec, J., Palacios-Rojas, N., & Fernie, A. R. (2015). Metabolite Profiles of Maize Under Drought Stress. Plant Physiology, 169(3), 1707-1723.
Xu, K., Xu, X., Fukao, T., Canlas, P., Maghirang-Rodriguez, R., Heuer, S., … Mackill, D. J. (2006). Sub1A is an Ethylene-Response-Factor-Like Gene that Confers Submergence Tolerance to Rice. Nature, 442(7103), 705-708. https://doi.org/10.1038/nature04920
Zandalinas, S. I., Mittler, R., Balfagón, D., Arbona, V., & Gómez-Cadenas, A. (2021). Plant Adaptations to the Combination of Drought and High Temperatures. Physiologia Plantarum, 171(1), 3-20.
https://doi.org/10.1126/science.218.4571.443
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2025. All Rights Reserved.