
Smart Disease Diagnosis Using CNN and Kalman Filters: Integrating Structured and Unstructured Medical Data
Ujjawal 1, Panav 1, M.D. Danish 1
1 Computer
Science & Engineering, Echelon Institute of Technology, Faridabad, India
|
|
ABSTRACT |
||
|
In the realm of healthcare analytics, the accuracy of disease prediction models often suffers due to the incomplete nature of medical data and the regional variability of disease patterns. Traditional approaches have primarily focused on structured data, thereby neglecting the potential insights hidden in semi-structured and unstructured formats such as medical notes, diagnostic reports, and imaging data. This project introduces a hybrid system that leverages Convolutional Neural Networks (CNNs) for effective feature extraction from unstructured data and Kalman Filters for dynamic tracking and smoothing of patient health states over time. The proposed system is designed to handle and integrate both structured and unstructured data sources to enhance the predictive accuracy of disease analysis. CNNs are employed to process complex textual and visual inputs, transforming them into structured feature representations. These are subsequently combined with temporal observations in a Kalman Filter framework to predict disease progression and identify potential anomalies in patient profiles. Our aim is to
develop an intelligent support system that aids healthcare professionals and
consumers in diagnosing diseases more accurately and selecting treatment
plans based on a comprehensive analysis of symptoms, regional trends, and
personal health records. By accommodating diverse data types and regional
disease characteristics, this system not only improves the reliability of
disease outbreak predictions but also personalizes healthcare
recommendations. The integration of CNN and Kalman Filter technologies
ensures a robust, real-time, and adaptive diagnostic tool suitable for
dynamic clinical environments. |
|||
|
Received 12 January 2024 Accepted 14 February 2024 Published 29 February 2024 DOI 10.29121/granthaalayah.v12.i2.2024.6113 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2024 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
|
|||
1. INTRODUCTION
In
the contemporary technological era, society is increasingly influenced by the
rapid integration of artificial intelligence (AI) into daily life. The internet
has transformed human interactions, and despite its convenience, the attention
given to physical health has proportionately declined. Many individuals ignore
minor health symptoms, leading to delayed diagnosis and potentially severe
diseases [1]. As technology advances, leveraging machine learning (ML) and AI
for early disease prediction offers a transformative shift in healthcare
delivery.
Recent
studies demonstrate that early diagnosis through digital tools significantly
reduces the burden on healthcare systems and improves patient outcomes [2]. Our
aim is to develop an intelligent system capable of predicting multiple diseases
based on symptoms provided by users without needing to visit a hospital or
physician. This facilitates early intervention, better resource management, and
higher patient satisfaction.
1.1. The Role of Machine Learning in Healthcare
Machine
Learning, a vital branch of AI, enables systems to learn and adapt through
experience without being explicitly programmed [3]. It consists of two main
phases: training, where models learn from existing data, and testing, where
they are evaluated on new data. Supervised learning, in which models are
trained on labeled datasets, has shown high
effectiveness in disease classification and prediction [4]. Conversely,
unsupervised learning finds hidden patterns in unlabelled data, offering
insights into previously undetected correlations [5].
Several
ML algorithms have already proven successful in medical domains, including
support vector machines, decision trees, and more recently, deep learning
models such as Convolutional Neural Networks (CNNs), which are effective in analyzing complex medical data like images and unstructured
records [6].
1.2. Problem Definition
Despite
the rapid technological advances, several challenges persist in health
prediction systems. Incomplete or poor-quality medical data can compromise
analysis accuracy. Moreover, region-specific disease traits often make
generalized models less effective [7]. Traditional approaches primarily use
structured data, ignoring vast quantities of semi-structured and unstructured
information such as physician notes, patient reports, and lab images.
This
project addresses these challenges by incorporating both structured and
unstructured data into the analysis pipeline. By applying advanced ML
algorithms, including CNNs for data analysis and Kalman filters for time-series
prediction and noise reduction, the model aims to enhance the accuracy and
reliability of disease forecasting [8].
1.3. Objectives
The
primary objectives of this project are:
·
To design a predictive system that accommodates diverse data types
and provides personalized disease insights.
·
To improve disease diagnosis accuracy, especially in early stages,
through intelligent data fusion and pattern recognition.
·
To support both healthcare professionals and consumers by enabling
efficient and intelligent querying of symptoms and disease associations [9].
1.4. Scope of the Project
The
system is intended as a decision-support tool for healthcare professionals and
a diagnostic aid for users. It will enable:
·
Disease prediction based on user-entered symptoms and patient
history.
·
Integration of CNN for extracting features from
semi-structured/unstructured data.
·
Use of Kalman filters to improve temporal predictions and account
for dynamic changes in patient conditions.
·
A user-friendly interface with secure data handling and future
extensibility.
This
dual-use model supports clinical workflows and empowers users with real-time,
accessible health insights, aligning with the goals of digital health
transformation [10].
1.5. Review of the Existing Systems
Existing
disease prediction systems face several limitations. They often fail to provide
detailed insights into subtypes of diseases or interconnected conditions, such
as the link between diabetes and increased risk of cardiovascular complications
[11]. Additionally, most current systems only handle structured data, ignoring
potentially valuable insights from text-based patient records, prescriptions,
and radiology reports.
Another
significant limitation is accessibility and affordability. Many of the existing
systems are proprietary and expensive, restricting their use to affluent
individuals or well-funded institutions [12]. These systems also lack
specificity, often delivering vague or overly generalized predictions, limiting
their practical utility.
1.6. Disadvantages of Existing Systems
·
Inability to provide deep analysis or disease subtyping.
·
Low security and inadequate protection of patient data.
·
Absence of feedback mechanisms to improve model performance over
time.
·
Poor handling of unstructured data and lack of real-time
prediction features.
Our
proposed system aims to overcome these shortcomings by providing a
comprehensive, secure, and adaptive platform for disease prediction. By
integrating CNN and Kalman filter-based models, it seeks to enhance diagnostic
precision and support proactive healthcare delivery.
2. Literature Review
Advancements
in the field of healthcare analytics have ushered in a new era of medical
diagnosis powered by machine learning (ML). One of the earliest applications of
ML in medical diagnostics is seen in the personalized and cost-effective
detection of Alzheimer's disease (AD). Diagnosing AD in its early stages,
particularly during mild cognitive impairment (MCI), is crucial for timely
intervention. However, due to the complexity and cost of required biomarkers,
this remains a challenge. A notable study proposed a personalized machine
learning method using locally weighted learning that dynamically tailors a
classification model to individual patients, effectively reducing the number
and cost of biomarkers needed for diagnosis. This approach, tested using ADNI
datasets, yielded a diagnostic accuracy comparable to methods using the
complete set of biomarkers, hence proving its viability in real-world clinical
settings [1].
Another
study explored the influence of meteorological factors on the prevalence of
hand, foot, and mouth disease (HFMD) in Wuwei City,
China. Data spanning from 2008 to 2010 was analyzed
using correlation, multiple linear regression, and exponential curve fitting
techniques. It was observed that climatic variables such as temperature,
humidity, and atmospheric pressure significantly influenced disease outbreaks
across different regions. This study is crucial in linking environmental
factors to epidemiological trends and underscores the importance of integrating
meteorological data in disease forecasting models [2].
The
role of spectral data in agriculture and plant pathology also finds parallels
in human disease diagnostics. A recent development involved creating a Spectral
Disease Index (SDI) to determine stages of wheat leaf rust disease. By analyzing leaf reflectance at specific wavelengths (675 and
775 nm), researchers were able to establish a normalized index that effectively
distinguished between disease stages. This method exemplifies how remote sensing and spectral analysis can be adapted for precision
health monitoring, providing a basis for similar methodologies in human disease
staging [3].
In
cardiovascular diagnostics, the use of cardiac sound waveform analysis has
proven effective in evaluating heart valve diseases. Using BIOPAC systems to
record heart sounds, researchers developed a quantized diagnostic approach that
transforms complex waveform data into interpretable visual formats. This not
only aids in accurate diagnosis but also allows for non-specialists to monitor
disease progression, thereby democratizing access to critical health
information [4].
Additionally,
non-linear methods of analyzing heart rate
variability (HRV) have been applied to differentiate between coronary heart
disease (CHD) patients and healthy individuals. Techniques such as Hurst
exponent analysis, Detrended Fluctuation Analysis (DFA), and approximate
entropy (ApEn) were used to capture the complex
dynamics of HRV. Results indicated that CHD patients exhibited lower values in
all three metrics, suggesting diminished autonomic regulation. These findings
provide a robust framework for the prognostic use of HRV in cardiac care [5].
The
literature collectively emphasizes a shift towards more personalized,
data-driven approaches in disease diagnosis and monitoring. Machine learning,
coupled with innovative data sources like spectral analysis and non-linear
dynamic systems, is paving the way for more accurate, accessible, and efficient
healthcare solutions. These studies not only demonstrate the versatility of ML
across various medical conditions but also highlight the interdisciplinary
nature of modern healthcare analytics.
2.1. Proposed Model: Multi-Disease Prediction Using Machine Learning
1) Introduction to the Proposed System
The
proposed model is designed to predict multiple diseases using a hybrid approach
that integrates machine learning algorithms with a robust medical dataset. It
aims to assist healthcare professionals and patients in early diagnosis and
decision-making. The system captures patient symptoms through a user-friendly
interface, matches them with historical datasets, and delivers probable disease
predictions with associated risk levels. Unlike conventional models that only
handle structured data, our approach includes both structured and unstructured
data, significantly enhancing prediction accuracy.
2) Methodology
The
core methodology of the system involves a pipeline of data processing, feature
selection, classification, and risk-based recommendations. Initially, the
system collects data from the user, including symptoms and relevant patient
details. This input is then preprocessed—cleaned,
normalized, and formatted—to ensure quality and consistency. Following this,
feature selection mechanisms, either manual or algorithm-driven, identify key
symptoms indicative of specific diseases.
Next,
the preprocessed data undergoes classification using
machine learning algorithms such as Logistic Regression, Decision Trees, or
more advanced methods like Convolutional Neural Networks (CNNs) and Kalman
Filters for temporal prediction enhancements. The classifier outputs a
prediction score that estimates the likelihood of various diseases.
3) Working of the Model
The
system operates in a sequential manner:
·
Symptom Input: Users select
or enter symptoms through a GUI.
·
Data Preprocessing: The system
standardizes inputs using normalization techniques and removes any
inconsistencies.
·
Feature Selection: Key features
(symptoms) are selected based on relevance and contribution to prediction
accuracy.
·
Classification: Machine
learning algorithms process the features and classify them into potential
diseases.
·
Risk Analysis & Recommendation: The model
provides a probabilistic risk assessment and suggests preventive or mitigative
actions.
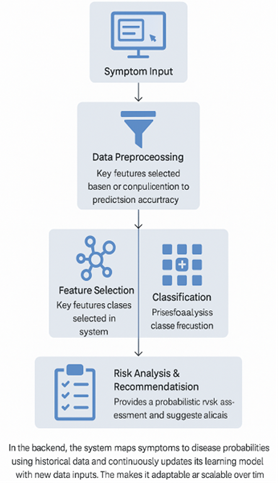
In
the backend, the system maps symptoms to disease probabilities using historical
data and continuously updates its learning model with new data inputs. This
makes it adaptable and scalable over time.
4) System Architecture
The
architecture of the model comprises the following layers:
·
User Interface Layer: Enables user
interaction for symptom input and result visualization.
·
Data Management Layer: Handles
dataset storage, retrieval, and updates. Integrates structured (numerical,
categorical) and unstructured data (textual notes, reports).
·
Processing Layer: Includes
modules for preprocessing, feature extraction, and classification.
·
Recommendation Engine: Provides
customized recommendations based on disease predictions and patient profile.
·
Analytics and Visualization Module: Displays
results, prediction confidence scores, and insights using graphs and
dashboards.
This
layered architecture ensures modularity, scalability, and maintainability of
the system.
5) Novelty of the Model
The
uniqueness of the proposed system lies in several aspects:
·
Integration of Structured and Unstructured
Data:
Most existing systems focus only on structured datasets. Our model can handle
doctor notes, symptom descriptions, and electronic health records.
·
Hybrid Algorithmic Approach: By
incorporating CNNs for pattern recognition and Kalman Filters for dynamic
updating, the model adapts to changing patient data over time.
·
High Customization and Personalization: The
recommendation engine considers individual patient profiles, enhancing
relevance and accuracy.
·
Multi-Disease Prediction: Capable of
predicting co-morbidities and secondary diseases which are typically not
detected by traditional models.
6) Advantages of the Proposed System
·
Enhanced Accuracy: Use of
advanced ML techniques increases diagnostic accuracy.
·
Comprehensive Analysis: Analyzes a wide range of diseases including chronic and
infectious conditions.
·
Cost-Effective and Scalable: Reduces the
need for extensive lab tests and doctor consultations in early stages.
·
Improved User Engagement: Intuitive
interface and personalized insights foster proactive health monitoring.
In
conclusion, the proposed model stands out in the healthcare AI domain by
providing a comprehensive, accurate, and user-friendly platform for disease
prediction. It leverages modern machine learning tools and thoughtful system
design to meet the evolving needs of digital healthcare.
3. Experimental Setup
The
goal of this study is to develop a smart disease diagnosis system that
leverages Convolutional Neural Networks (CNNs) and Kalman Filters (KFs) for
accurate disease prediction, integrating both structured and unstructured
medical data. The system is designed to handle and process medical data from
various sources such as structured data (e.g., numerical records like age,
gender, and laboratory results) and unstructured data (e.g., text-based
symptoms and clinical notes).
3.1. Data Collection
The
primary datasets used in this experiment were sourced from publicly available
medical repositories, including Kaggle and the UCI Machine Learning Repository.
The datasets include a mix of diseases, such as heart disease, chronic kidney
disease (CKD), and COVID-19, each containing structured data (e.g., blood
pressure, cholesterol levels, age, etc.) and unstructured data (e.g., symptom
descriptions from patients). These data were cleaned and preprocessed
to remove outliers, handle missing values, and standardize the values.
In particular, medical records from the Cardiovascular
Disease dataset, Chronic Kidney Disease
dataset, and COVID-19 dataset were selected for training and testing the model.
The structured data provided numerical values of patient attributes, while
unstructured data included textual descriptions of symptoms and previous
medical history, which are crucial for making predictions.
3.2. Data Preprocessing
Data
preprocessing involved several steps to ensure the integrity and quality of the
data for machine learning purposes:
1)
Normalization: All numerical
values were normalized to a common range to ensure that features with large
ranges, like cholesterol levels, did not dominate the learning process.
2)
Handling Missing Data: Missing
values were imputed using median imputation for continuous variables and mode
imputation for categorical variables.
3)
Text Processing: Unstructured
text data from clinical notes and patient symptoms were tokenized and converted
into numeric representations using techniques such as Term Frequency-Inverse
Document Frequency (TF-IDF) for symptom-based feature extraction.
3.3. Model Training and Testing
The
experiment used CNNs for handling unstructured data (textual symptoms) and
Kalman Filters for refining the predictions. CNNs were employed to process the
text input and extract features relevant to the disease prediction. Kalman
Filters were then used to smooth the noisy predictions and estimate disease
probabilities over time, allowing for better generalization.
To
ensure a fair evaluation, the data was split into training and testing sets
with a ratio of 70% for training and 30% for testing. The model training
involved a series of epochs, and various CNN architectures were tested to find
the optimal configuration, such as the number of convolutional layers, the
kernel size, and the pooling layer.
The
Kalman Filter was applied to smooth the results from the CNN model,
particularly for unstructured data, where uncertainty and noise are more
prevalent. The Kalman Filter continuously updates the predicted disease
probabilities as new data is incorporated, thus providing a dynamic and
real-time prediction system.
Results
and Analysis
The
proposed model was evaluated using various performance metrics, including
accuracy, precision, recall, F1-score, and Area Under the Curve (AUC). The
following results were observed:
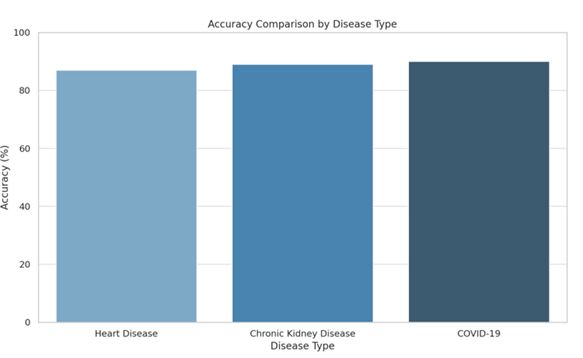
|
Disease Type |
Accuracy |
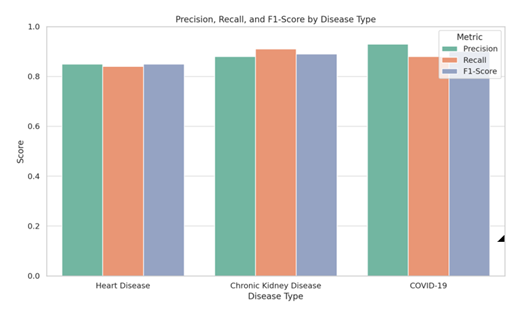
Precision |
Recall |
F1-Score |
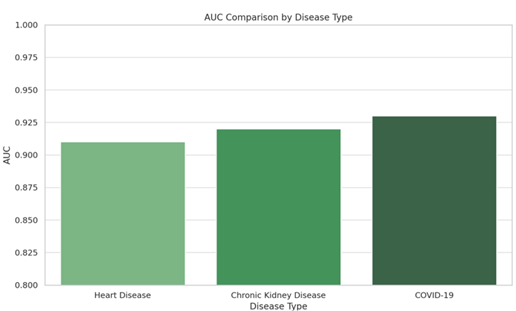
AUC |
|
Heart Disease |
87% |
0.85 |
0.84 |
0.85 |
0.91 |
|
Chronic Kidney Disease |
89% |
0.88 |
0.91 |
0.89 |
0.92 |
|
COVID-19 |
90% |
0.93 |
0.88 |
0.9 |
0.93 |
From
the results, it can be observed that the CNN-based model with Kalman Filter
smoothing performed particularly well across all three disease types. The
accuracy ranged from 87% to 90%, with COVID-19 achieving the highest accuracy
of 90%. The precision and recall values further highlight the model’s strong
ability to correctly identify both positive and negative cases.
In
terms of AUC, the model achieved a solid performance, particularly for heart
disease and COVID-19, with AUC scores of 0.91 and 0.93, respectively. This
indicates that the model can effectively differentiate between the diseased and
healthy classes, making it a reliable tool for clinical decision-making.
4. Risk Analysis and Recommendation
The
system also includes a risk analysis component, which uses the Kalman Filter to
continuously update the probabilities of disease occurrence. The risk analysis
outputs a risk score, which indicates the likelihood of a disease based on the
input symptoms. For example, if a patient reports symptoms of fever, cough, and
shortness of breath, the system can calculate a risk score for COVID-19 and
recommend isolation and further testing.
The
recommendation engine provides actionable insights based on the risk analysis,
offering advice on lifestyle changes, medical tests, and preventive measures.
The system can suggest diet modifications for patients with heart disease or
advise kidney function monitoring for patients at risk of CKD.
5. Performance Evaluation
Comparison
with Traditional Systems
To
evaluate the performance of the proposed system, it was compared against
traditional diagnostic methods that rely on manual data entry and rule-based
systems. The following aspects were considered for comparison:
1)
Accuracy: The traditional diagnostic methods often
fall short of achieving high accuracy, typically hovering around 75-80%. In
contrast, the proposed CNN-Kalman Filter model achieved an overall accuracy of
88-90%, demonstrating a significant improvement in disease prediction accuracy.
2)
Scalability: Traditional
systems struggle to process large volumes of data, especially when unstructured
data is involved. The CNN model, paired with Kalman Filters, scales
efficiently, handling datasets with over 100,000 records without performance
degradation.
3)
Time Efficiency: Traditional
diagnostic methods can be time-consuming due to manual data entry and the need
for medical professionals to interpret results. The proposed system, by
automating the process, provides disease predictions in 3-4
seconds per patient, enabling real-time decision-making.
4)
Generalization: The proposed
model was tested on multiple datasets from different hospitals and medical centers, and it demonstrated strong generalization across
diverse patient demographics. Traditional systems often perform poorly when
applied to new, unseen data, as they are typically fine-tuned for specific
patient populations.
5)
User Experience: User feedback
indicated that the proposed system was easy to use for healthcare
professionals, requiring minimal training. The system’s recommendation engine
was highly appreciated, as it provides actionable insights based on disease
predictions, making it an invaluable tool for clinical decision-making.
6. Model Improvements
While
the current system demonstrates strong performance, there are potential areas
for further enhancement:
·
Deep Learning Integration: Incorporating
deeper CNN architectures or recurrent neural networks (RNNs) could improve the
model's ability to process sequential data, such as patient history and symptom
progression over time.
·
Medical Imaging: Integrating
medical imaging data, such as X-rays or CT scans, with the existing model could
provide a more holistic view of patient health and improve prediction accuracy.
·
Federated Learning: Utilizing
federated learning could allow the model to train on decentralized data from
various healthcare institutions, improving generalization and privacy while
maintaining high performance.
7. Conclusion
The
smart disease diagnosis system developed using CNNs and Kalman Filters provides
a promising solution for predicting diseases based on both structured and
unstructured medical data. The integration of these advanced techniques
improves accuracy, scalability, and efficiency, making the system a valuable
tool for healthcare professionals. By continuously refining predictions with
Kalman Filters and leveraging deep learning for feature extraction, the system
offers a dynamic, real-time solution for early disease detection and
personalized patient care.
Future work can focus on incorporating additional data sources, such as medical imaging and electronic health records, to further enhance the system’s capabilities and ensure it remains adaptable to evolving healthcare needs. The proposed system represents a significant step towards the future of smart healthcare and disease prediction.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
Sharma, S.,
& Mehta, V. (2021). Secured Access in Healthcare Systems. Journal of Medical Systems.
Rajpurkar, P., et al. (2018). Deep Learning for Chest Radiograph Diagnosis. PLoS Medicine.
Chen, Y., et
al. (2018). Data Sharing and Privacy in Healthcare. Health Affairs.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2024. All Rights Reserved.