
A review study on the effect of oral curcumin on breast cancer with the reference of clinical and biochemical markers
Priya Pandey 1, Shaily Tyagi 2![]() , Mukesh Pandey 3
, Mukesh Pandey 3
1 Associate
Professor, in Greater Noida Institute of Technology, India
2 Assistant
Professor, Quantum University, Roorkee, India
3 Assistant Professor, Shri Guru Ram Rai University, Dehradun, India
|
|
ABSTRACT |
||
|
As one of the
most common cancers in the world, breast cancer requires ongoing studies into
efficient treatment approaches. The polyphenol curcumin, which comes from
Curcuma longa, has attracted a lot of attention because of its possible
anti-cancer effects. This review assesses the impact of oral curcumin on
biochemical markers and clinical factors related to breast cancer. To
evaluate curcumin's effects on tumour growth, oxidative stress, inflammatory
markers, and patient survival outcomes, we look at preclinical and clinical
research. Breast cancer is a complex illness that is impacted by
environmental, hormonal, and hereditary variables. Currently available
therapeutic options include radiation, chemotherapy, surgery, and targeted
medicines; nevertheless, these methods frequently have serious adverse
effects. Curcumin is a prospective adjuvant therapy for the treatment of
breast cancer because of its anti-inflammatory, antioxidant, and
anti-proliferative qualities, which have been studied. The effectiveness of
oral curcumin in modifying the biochemical and clinical indicators linked to
breast cancer is examined in this review. In order to enhance patient
outcomes, new adjunct medicines must be investigated because breast cancer
continues to rank among the world's leading causes of cancer-related
mortality. The bioactive polyphenol curcumin, which comes from Curcuma longa,
has drawn a lot of interest because of its anti-inflammatory, antioxidant,
and anticancer qualities. This study evaluates clinical characteristics and
biochemical markers in preclinical and clinical research to investigate the
impact of oral curcumin supplementation on breast cancer. There is evidence
that curcumin inhibits metastasis, reduces angiogenesis, and induces
apoptosis via modulating important molecular pathways such as PI3K/Akt,
NF-κB, and MAPK signaling. Curcumin has also been shown to reduce
oxidative stress markers, tumor biomarkers like CA 15-3 and CEA, and
inflammatory cytokines like IL-6 and TNF-α. There are still issues with
its absorption and the best ways to dose it, despite clinical trials showing encouraging
outcomes in terms of enhancing patient quality of life and slowing tumor
growth. Subsequent investigations ought to concentrate on augmenting
curcumin's medicinal effectiveness via sophisticated formulations and
combination treatments. |
|||
|
Received 18 February
2025 Accepted 22 March 2025 Published 16 April 2025 Corresponding Author Shaily
Tyagi, shailytyagi664@gmail.com DOI 10.29121/granthaalayah.v13.i3.2025.6008 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2025 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Clinical Parameter, Breast Cancer, Inflammation,
Apoptosis, Carcinogenesis |
|||
1. INTRODUCTION
One of the biggest causes of cancer-related death for women globally is still breast cancer. Significant obstacles still exist, including as medication resistance, unfavorable side effects, and recurrence, even with improvements in traditional treatments like surgery, chemotherapy, radiation, and targeted therapies. In order to improve therapeutic efficacy and lower toxicity, there has been an increase in interest in complementary and alternative medicines, especially those made from natural chemicals. Because of its strong anti-inflammatory, antioxidant, and anticancer effects, curcumin—a polyphenolic chemical obtained from the rhizome of Curcuma longa, or turmeric—has drawn a lot of interest in cancer research. According to a number of preclinical and clinical investigations, curcumin inhibits apoptosis, cell cycle regulation, angiogenesis, and metastasis, among other molecular pathways, to produce its anticancer effects. The purpose of this review is to assess how oral curcumin affects breast cancer, with an emphasis on biochemical and clinical indicators. This study will evaluate the therapeutic potential of curcumin in patients with breast cancer by reviewing the literature and taking into account its effects on important biomarkers such oxidative stress indicators, tumor suppressor genes, inflammatory cytokines, and signaling pathways implicated in the evolution of the disease. In addition, the pharmacokinetics and bioavailability of oral curcumin will be examined, as well as the difficulties and possible solutions to enhance its therapeutic effectiveness.
Radiation therapy, chemotherapy, surgery, and targeted therapy are some of the treatment options available for breast cancer, which is still one of the most common and difficult cancers in the world. But worries about side effects, tumor recurrence, and medication resistance have led scientists to look into natural substances that have anticancer qualities. One such substance is curcumin, a polyphenol that comes from the turmeric plant Curcuma longa. It has shown strong anticancer properties, especially against breast cancer.
Curcumin's anti-inflammatory, antioxidant, antiproliferative, and pro-apoptotic properties on cancer cells have been the subject of much research. When taken orally, curcumin affects tumor development, metastasis, and chemoresistance by interacting with several molecular pathways implicated in the progression of breast cancer.
2. Mechanism of action
2.1. Apoptpsis induction and inhibition of cell proliferation
Through the downregulation of pro-survival signaling pathways such PI3K/Akt/mTOR and MAPK/ERK, curcumin inhibits the development of
Breast cancer cells.By downregulating anti-apoptotic proteins including Bcl-2 and upregulating p53,
Bax, and caspases, it improves apoptotic pathways.
2.2. Antioxidant and anti inflammatory properties
One known factor contributing to the development of cancer is chronic inflammation. NF-κB, a crucial regulator of inflammation and cancer cell survival, is inhibited by curcumin.
By eliminating free radicals and increasing the activity of antioxidant enzymes like glutathione peroxidase and superoxide dismutase (SOD), it lowers oxidative stress.
2.3. Angiogenesis and METASTASIS SUPPRESSION
Targeting VEGF (vascular endothelial growth factor) and HIF-1α (hypoxia-inducible factor-1α), which are essential for the development of blood vessels in tumors, curcumin prevents tumor angiogenesis.
By modifying matrix metalloproteinases (MMPs), which are involved in the breakdown of extracellular matrix, a crucial stage in tumor spread, it lowers the metastasis of breast cancer.
2.4. The alteration of hormonal pathways in breast cancer that is estrogen receptor-positive (ER+)
By affecting estrogen receptor (ER) signaling and inhibiting the growth of ER+ breast cancer cells,
Curcumin function as a natural estrogen modulator.It may help treat hormone-dependent breast cancer by inhibiting aromatase, the enzyme that produces estrogen.
2.5. Increasing therapeutics efficacy and overcoming chemoresistance
Curcumin inhibits survival pathways and decreases drug efflux mechanisms, making breast cancer cells more susceptible to chemotherapy drugs such as doxorubicin, paclitaxel, and cisplatin.
By raising the amounts of reactive oxygen species (ROS) in cancer cells, it also improves the efficacy of radiation therapy.
3. Role of curcumin in immunotherapy
Curcumin has been shown to cause modulation of tumor associated immunological response mechanism thus, remodeling the tumor-linked immunosuppressive surrounding microenvironment. This indicates its true potential in aiding as an adjunct in cancer immunotherapy.
A tumorous microenvironment is very complex as well as heterogeneous in nature. O'Donnell et al has classified this tumor micro-environment into four types based upon burden of a mutation in a tumor along with degree and sub-groups of the tumor infiltrating
T lymphocytic cell population. Thus, various types of immunological microenvironments which can suppress and also, cause enhancement of a tumor microenvironment may as well correspond to specific tumor types along with different types of immunotherapeutic that can be associated with a particular tumor type Rachman, Torchilin et al. 2016.
Thus, a specific variety of immunotherapy can be administrated as per different varieties of immunological micro-environment associated with any tumor. Various immune-suppressive cells within a tumor micro-environment may be capable of facilitating progression of various tumors through generation of a tumor-specific microenvironment that causes suppression of immunological response.
Evidence from previously published research work has demonstrated that in early stages of any tumor, both the innate and adaptive immunities play an active role in immunological surveillance, thereby, causing suppression of tumor genesis, that can be transient in nature Saxena and Hussain et al. 2013.
Various tumor associated antigenic molecules have been considered a necessary part or component that helps in stimulation of tumor associated immunity response. The innate immunological cells like- Dendritic cells, macrophages and Natural Killer cells act by recognizing various antigens associated with a tumor which cause stimulation of cytotoxicological cellular effects that can kill these tumor cells.
These antigen-presentation cells also contain tumor derived antigens in order to bind to adaptive immunological cells, for example, cytotoxic T-cell lymphocytes or T- helper cells (Th1) to cause stimulation of specific destructive pathways active against these tumors.
A regenerated tumor elicits memories of adaptive immunity arm that may exert an identical destructive effect on these tumorous cells by mediation of escape from immunological response by alteration of composition of various immune cells infiltrating any tumor Sepehri, Attari et al. 2017. This occurs by recruitment of numerous immune-suppressive cytokines. These cytokines because induction by an increase in synthesis of Treg cells, M2 macrophages (which show a tolerance towards antigenicity) and dendritic cells. Also, there is reduction in infiltration of cytotoxic T cell Lymphocytes and Natural Killer (NK) cells which results in loss of immunological surveillance mechanism of a tumor.
Secondly, the tumor associated antigens may be non tumor specific and may act by binding with normal body cells as they also may demonstrate similar antigen which mediates the innate immunological mechanism of tolerance that can lead to dys functioning of host immunity enabled recognition of these tumor associated antigenic molecules.
Thirdly, an absence of a major histocompatibility complex (MHC) class I molecule on surfaces of tumor cells may cause inability in immunological recognition of the CD8+cytotoxic T lymphocytic cells in mediating the immunological tolerance.
Also, modifications in post transcription process of few of these tumor specified antigenic genes, for example, GIL-1 seen in multiple myeloma and hPMS1 present in oral Squamous cell carcinoma, might result in the down-regulation of their expressions or total silencing.
“Immunoediting” is a type of process of Darwinian selection. It comprises of three stages-
· Elimination (also termed as „immunosurveillance‟)
· Cancer dormancy which is also termed as „equilibrium‟.
· Immunological escape. These three stages are collectively termed as 3
One of the main mechanisms by which curcumin modify tumor microenvironment is by modulating functions of various cytokines. Thus, while the gradual structuring of a tumor associated immune-suppressed microenvironment is being achieved, it has been shown that a variety of cytokines play an important role in regulation of composition and proportion of immune cellular component responsible for promotion of growth of any cancer along with cellular components responsible for suppression of any cancer (Pramanik, Makena et al. 2018).
Curcumin acts by inhibiting a tumor which besides facilitating secretion of cytokines which help in cancer growth also causes inhibition of cytokines‟ secretion responsible for suppression of various cancers. Therefore, remodeling of immune-suppressive microenvironment of any tumor occurs by this mechanism.
Interleukin-2 (IL‑ 2) has a dual function of causing the stimulation and inhibition of immune response exerted by a tumor mass. The high affinity Inetrleukin‑ 2 receptor (IL‑ 2R) is overtly expressed over surfaces of Regulatory T (Treg) cells; IL‑ 2 has a tumor promoting activity which regulates these Treg cells. Curcumin acts by direct inhibition of an interaction occurring between IL‑ 2 and IL‑ 2Rα (also termed as, CD25). This results in inhibition of activity of the Treg cells Sanchez, Simon et al. 2010.
This suppressive activity results in malfunctioning of the JAK/STAT signaling pathway. It has also been shown to down regulate TGF‑ β and IL‑ 10 levels in the T regulatory cells. This causes a reduction in their capability for stimulation of T-reg cells which results in a decrease in Treg cellular infiltration within tumors.
Increase in expression levels of Interlekin‑ 6 in triple negative breast carcinoma has been considered to cause mediation of the immunosuppressive tumor microenvironment which leads to failure of vaccine therapy directed against a tumors failure Morrone, Battastini et al. 2015. Curcumin is also a highly specific inhibitor of this cytokine. Combination of curcumin with Listeriaat‑ Mage‑ b, a breast cancer vaccine has demonstrated results with decreased expression in interleukin‑ 6 levels along with a concomitant rise in expressions of Interleukin‑ 12 and interferon- γ (IFN‑ γ) levels. These results have demonstrated increase in anti‑ tumorogenic efficacy when compared with use of only the vaccine.
A part from affecting adaptive immunity arm, Curcumin has been shown to regulate the proliferation along with apoptosis of T lymphocytes. It also causes influence on the Natural Killer mediated innate cellular immunity.In a study involving breast carcinoma, exosome that are secreted by tumors „cells have been found to abolish activity of the NK cells through interleukin‑ 2 by inhibition of JAK3/STAT-5 signaling pathway.
It may also cause inhibition of cytotoxic effects of exosome on circulating immunity cells by disruption of the ubiquity-protease system along with an increase in ubiquitinated exosome proteins interfering with functioning of normal exosome proteins.
Curcumin also affects the tumor microenvironment by mediating transformation of M2 type macrophage into M1-type macrophages. The tumor associated macrophages (TAMs) have been classified as follows- a) inducible nitric oxide synthesis (iNOS); b) M1 (+) macrophages and c) ARG1+ M2 macrophages based upon their functions according to the binary classification system proposed by Mills et al.
4. Safety parameters tested for curcumin usage
In a Phase I clinical trial, 15 subjects diagnosed with refractory advanced stage colorectal cancer were administered various dosages of curcumin which ranged from
0.45 grams to 3.6 grams on daily basis for a duration of 4 months in order to determine its systemic effects. Based on these findings, a good drug safety profile was defined. Systemic biomarkers indicating the potential activity of curcumin have indicated a modulation of transcription of COX-2, which has been linked to prevention of cancers along with their treatment. Based upon these findings, a daily dosage of 3.6 grams of curcumin was recommended for further Phase II clinical trials Zanotto-Filho et al. 2015.
Oral route of administration of curcumin has been shown to exhibit good tolerance without any toxic effects at dosages of 8 grams per day for up to a period of 18 months. Though this molecule has poorly absorption which has been detected by low levels (in nano-grams) of circulating curcumin which is detectable in steady state equilibrium, its biological activities are also evident as well.
Pre-clinical study data have suggested that curcumin indeed shows potential activity against all forms of pancreatic carcinomas; however, higher levels of this drug‟s exposure should be achieved for its maximal effectiveness M. Battastini, Forcelini et al. 2003.
Multiple cellular pathways which involve the activation of NF-ƙβ, signaling of EGFR and PI3/AKT/mTOR pathways, expression of STAT3, activation of cascade involving MAP kinase and angiogenesis mediated by VEGF have been demonstrated as deregulated in head and neck SCC. These molecules represent potential cancer therapeutic targets. Some promising results from these targeted therapies have been obtained but complexities of interactions between these signaling pathways contribute to limited clinical response that is observed with use of a single agent-based biological therapy.
Thus, in addition to being a natural plant derivative, curcumin has both non-toxic properties and variety of inhibitory effects on pathways that are involved in process of carcinogenesis and tumorigenesis Veena and Wang MB, Srivatsan et al. 2006. The lack of systemic toxicity and broad-reaching mechanism of activity makes it the agent suited as use in adjuvant therapy for head and neck cancers which are slowly exhibiting resistance to the current therapies that are routinely used.
Since curcumin is a hydrophobic molecule, it cannot be administered through intravenous route. It has a lypophilic nature; it may be encapsulated within liposomes. These preparations allow intravenous administration of this drug which can lead to high levels of circulating curcumin.
There are several limitations in administering curcumin for therapeutic use in clinical oncological practice due to factors like- a) low bio-availability, b) disproportionate effects of drug dosage and c) poor water solubility Gilvary, D. Coppola et al. 2017.
Hence, to solve these limitations, there have been increases in numbers of analytical studies that have focused up on- a) enhancement of host‟s bodily tolerance towards this drug, b) improvement in targeting of the drug delivery to tissues, c) overcoming low levels of its bioavailability and d) achievement of a significantly higher therapeutic effectively of this molecule. Also, there are numerous nano curcumin based formulations which have been recently introduced and have demonstrated considerable improvement in the anti-carcinogenic effects of curcumin molecule Mamoru, Rayers et al.2013. Therefore, it can be considered that curcumin may serve a highly important and significant role in cancer prevention and treatment in future of cancer management.
Figure1
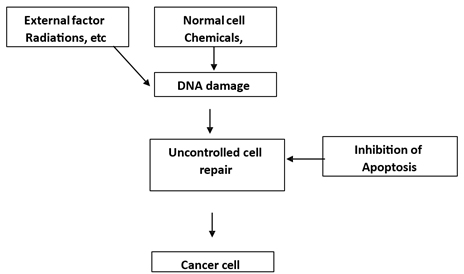
|
Figure 1 Diagrammatic Representation on How Cancer Takes Place |
The combined effects of oral curcumin and docetaxel treatment in individuals with advanced and metastatic breast cancer were examined in a noteworthy clinical trial. For seven days throughout each treatment cycle, patients in this trial were given up to 8 grams of curcumin each day. The findings showed that curcumin was typically well tolerated, while the main toxicities linked to docetaxel were neutropenia and leukopenia. The high number of capsules needed for the dosage caused two individuals to stop taking curcumin.
Curcumin or diferuiloylmethane is a polyphenolic compound, extracted from plant Curcuma longa has been used in Indian system of medicine because of its anti- inflammatory property, anti-tumor property. Curcumin is a brightly yellow colored chemical and a member of the ginger family, Zingiberaceae. It is also used in cosmetics; good flavoring and food coloring. Curcumin is generally recognized as safe by the Food and Drug Administration (FDA) Ali. Ahmad et al. 2010.
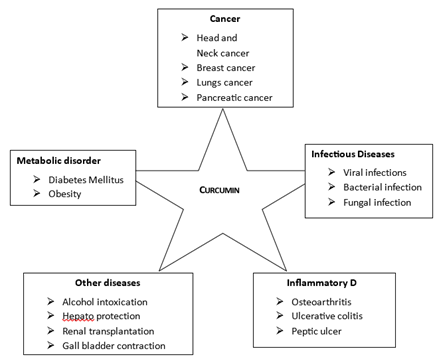
Clinical parameter
Imaging methods, physical examinations, and patient histories serve as the main foundation for clinical indicators. Among the important clinical indicators are:
Palpable Lump: The initial indication of a tumor is frequently a firm, immobile mass in the breast tissue.
Skin Changes: Underlying cancer may be indicated by skin thickening, dimpling, or redness (peau d'orange look).
Bloody or serous discharge from the breasts could be a sign of an underlying malignancy.
Lymph Node Involvement: Swollen lymph nodes in the supraclavicular or axillary regions indicate metastases.
Pain and Tenderness: While most aggressive forms of breast cancer are painless, some might cause pain.
Imaging Results: Important details about the size, shape, and kind of tumor are revealed by mammograms, ultrasounds, and magnetic resonance imaging (MRI).
Biochemical indicators
Breast cancer can be detected by biochemical indicators, such as tumor markers, which are molecules Present in tissue, blood or other body fluid.
Predictive and prognostic biomarkers
Status of hormone receptor: The response to hormone therapy is determined by the indicators known as the estrogen receptor (ER) and progesterone receptor (PR). In general, ER/PR-positive cancers react well to endocrine therapy and have a better prognosis.
The receptor for human epidermal growth factor: Despite being more aggressive, HER2-positive cancers react favorably to targeted treatments such as trastuzumab (Herceptin).
Researcher had found that various pathway had been targeted by this curcumin analogues which made this product so versatile.
Its most important active ingredient is curcuminoid. Curcuminoid are phenolic compounds commonly used as aspice, pigment and additive also utilized as a therapeutic agent used in several foods.
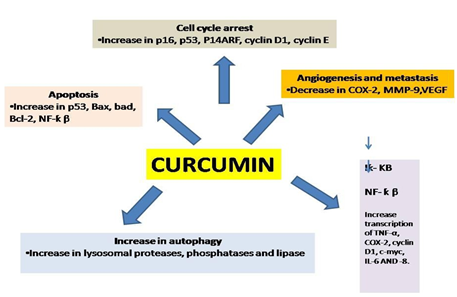
Comprehensive research over the last century has revealed several important functions of curcuminoid. Various preclinical cell culture and animals studies suggest that curcuminoid Comprehensive research over the last century has revealed several important functions of curcuminoid. Various preclinical cell culture and animals studies suggest that curcuminoid have extensive biological activity as an antioxidant, neuroprotective, antitumor, anti-inflammatory, anti- acidogenic, radio protective and arthritis. Different clinical trials also suggest a potential therapeutic role for curcuminoid in numerous chronic diseases such as colon cancer, lungs cancer, breast cancer, inflammatory bowel diseases Guo, Shu et al. 2015.
Natural agents:
Agents which are obtained from plant or nature or through natural things are called natural agents like garlic glutathione etc.
Many treatment like chemotherapy, radiotherapy are existing but the drawback with such therapies include they target one target at a time and had lot of side effects due to which researcher are now focusing to search a multi targeting molecules that can target many receptor at a time but this therapy again process some undesirable side effect Irving, Iwuji et al. 2015.
So now scientists are trying to use our natural agents which are being used to treat various disorders from an era. Curcumin a natural ingredient obtained from plant Curcuma longa belongs top such class of drugs.
Curcumin is cultivated in several tropical and sub tropical part of the world. The curcumin possess several beneficial properties. Curcumin or diferuiloylmethane is a polyphenolic compound, extracted from plant Curcuma longa has been used in Indian system of medicine because of its anti- inflammatory property, anti tumor property. This compound is hydrophobic in nature Mirzaei, Naseri et al. 2016.
5. Conclusion
Because of its diverse anticancer actions, oral curcumin offers a viable natural therapy approach for breast cancer. It can improve the effectiveness of traditional treatments, decrease inflammation, stop metastases, and suppress tumor growth. Its limited bioavailability is still a problem, though, and requires sophisticated formulations for clinical use. To determine uniform dosages and maximize its therapeutic potential in the treatment of breast cancer, more clinical trials are necessary.
In order to diagnose, prognosticate, and treat breast cancer, clinical and biochemical markers are crucial. Biochemical indicators provide information about tumor biology, treatment response, and disease progression, whereas clinical markers offer preliminary indications. Personalized therapy approaches are being improved by advances in molecular diagnostics, which is improving patient outcomes.
Numerous clinical and biochemical indicators have shown that curcumin, the main ingredient in turmeric, has encouraging potential as a supplemental treatment for breast cancer. According to studies, curcumin inhibits tumor growth, induces apoptosis, suppresses inflammatory pathways, and interacts with important molecular targets like NF-κB, STAT3, and PI3K/AKT to provide its anticancer effects. Clinical trials have shown that curcumin, especially when combined with traditional therapy, can reduce tumor development and improve inflammatory markers (e.g., TNF-α, IL-6, and CRP).
Clinical and biochemical signs suggest oral curcumin's promising potential as an adjuvant treatment therapy for breast cancer. While biochemical analyses demonstrate curcumin's capacity to modulate important molecular pathways involved in the development of cancer, such as NF-κB, PI3K/Akt, and apoptotic mechanisms, clinical studies indicate that curcumin can improve treatment efficacy, decrease tumor progression, and improve patient quality of life. Its function in cancer care is further supported by its anti-inflammatory, antioxidant, and anti-metastatic qualities. Bioavailability is still a major problem, though, and in order to maximize its therapeutic use, more research into innovative delivery methods and combination treatments is required. To verify curcumin's effectiveness and create uniform dosage schedules for the treatment of breast cancer, further extensive randomized controlled trials are required.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
Aceto, N., Sausgruber, N., Brinkhaus, H., Gaidatzis, D., Martiny-Baron, G., Mazzarol, G., et al. (2012). Tyrosine phosphatase SHP2 promotes breast cancer progression and maintains tumor-initiating cells via activation of key transcription factors and a positive feedback signaling loop. Nature Medicine, 18 (4), 529. https://doi.org/10.1038/nm.2645
Aggarwal, B. B. (2008). Prostate cancer and curcumin: Add spice to your life. Cancer Biology & Therapy, 7 (9), 1436–1440. https://doi.org/10.4161/cbt.7.9.6659
Akram, M., Shahab-Uddin, A. A., Usmanghani, K., Hannan, A., Mohiuddin, E., & Asif, M. (2010). Curcuma longa and curcumin: A review article. Romanian Journal of Biology – Plant Biology, 55 (2), 65–70.
Aktas, C., Kanter, M., Erboga, M., & Ozturk, S. (2012). Anti-apoptotic effects of curcumin on cadmium-induced apoptosis in rat testes. Toxicology and Industrial Health, 28 (2), 122–130. https://doi.org/10.1177/0748233711407242
Anand, P., Kunnumakkara, A. B., Newman, R. A., & Aggarwal, B. B. (2007). Bioavailability of curcumin: Problems and promises. Molecular Pharmaceutics, 4 (6), 807–818. https://doi.org/10.1021/mp700113r
Anderson, W. F., Chatterjee, N., Ershler, W. B., & Brawley, O. W. (2002). Estrogen receptor breast cancer phenotypes in the Surveillance, Epidemiology, and End Results database. Breast Cancer Research and Treatment, 76 (1), 27–36. https://doi.org/10.1023/a:1020299707510
Arablou, T., & Kolahdouz-Mohammadi, R. (2018). Curcumin and endometriosis: Review on potential roles and molecular mechanisms. Biomedicine & Pharmacotherapy, 97 (1), 91–97. https://doi.org/10.1016/j.biopha.2017.11.063
Bharti, A. C., Donato, N., Singh, S., & Aggarwal, B. B. (2003). Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor–κB and IκBα kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood, 101 (3), 1053–1062. https://doi.org/10.1182/blood-2002-05-1320
Bhavanishankar, T., Shantha, N., Ramesh, H., Murthy, I., & Murthy, S. (1980). Toxicity studies on turmeric (Curcuma longa): Acute toxicity studies in rats, guinea pigs, and monkeys. Indian Journal of Experimental Biology, 18 (1), 73–75.
Choi, H. Y., Lim, J., & Hong, J. H. (2010). Curcumin interrupts the interaction between the androgen receptor and Wnt/β-catenin signaling pathway in LNCaP prostate cancer cells. Prostate Cancer and Prostatic Diseases, 13 (4), 343–349. https://doi.org/10.1038/pcan.2010.26
Cort, A., Timur, M., Ozdemir, E., Kucuksayan, E., & Ozben, T. (2012). Synergistic anticancer activity of curcumin and bleomycin: An in vitro study using human malignant testicular germ cells. Molecular Medicine Reports, 5 (6), 1481–1486. https://doi.org/10.3892/mmr.2012.848
Dorai, T., Gehani, N., & Katz, A. (2000). Therapeutic potential of curcumin in human prostate cancer—I. Curcumin induces apoptosis in both androgen-dependent and androgen-independent prostate cancer cells. Prostate Cancer and Prostatic Diseases, 3 (2), 84–93. https://doi.org/10.1038/sj.pcan.4500399
Farombi, E. O., Abarikwu, S. O., Adedara, I. A., & Oyeyemi, M. O. (2007). Curcumin and kolaviron ameliorate di-n-butylphthalate-induced testicular damage in rats. Basic & Clinical Pharmacology & Toxicology, 100 (1), 43–48. https://doi.org/10.1111/j.1742-7843.2007.00005.x
García-Aranda, M., & Redondo, M. (2017). Protein kinase targets in breast cancer. International Journal of Molecular Sciences, 18 (12), 2543. https://doi.org/10.3390/ijms18122543
Hallman, K., Aleck, K., Dwyer, B., Lloyd, V., Quigley, M., & Sitto, N. (2017). The effects of turmeric (curcumin) on tumor suppressor protein (p53) and estrogen receptor (ERα) in breast cancer cells. Breast Cancer (London, England), 9, 153. https://doi.org/10.2147/BCTT.S125783
Heinlein, C. A., & Chang, C. (2004). Androgen receptor in prostate cancer. Endocrine Reviews, 25 (2), 276–308. https://doi.org/10.1210/er.2002-0032
Jason, C. Y., & Formenti, S. C. (2018). Integration of radiation and immunotherapy in breast cancer: Treatment implications. The Breast, 38 (1), 66–74. https://doi.org/10.1016/j.breast.2017.12.005
Jobin, C., Bradham, C. A., Russo, M. P., Juma, B., Narula, A. S., Brenner, D. A., et al. (1999). Curcumin blocks cytokine-mediated NF-κB activation and proinflammatory gene expression by inhibiting inhibitory factor I-κB kinase activity. Journal of Immunology, 163 (6), 3474–3483.
Karunagaran, D., Rashmi, R., & Kumar, T. (2005). Induction of apoptosis by curcumin and its implications for cancer therapy. Current Cancer Drug Targets, 5 (2), 117–129. https://doi.org/10.2174/1568009053202081
Lai, H.-W., Chien, S.-Y., Kuo, S.-J., Tseng, L.-M., Lin, H.-Y., Chi, C.-W., et al. (2012). The potential utility of curcumin in the treatment of HER-2-overexpressed breast cancer: An in vitro and in vivo comparison study with herceptin. Evidence-Based Complementary and Alternative Medicine, 1 (1), 1. https://doi.org/10.1155/2012/486568
Li, J., Xiang, S., Zhang, Q., Wu, J., Tang, Q., Zhou, J., et al. (2015). Combination of curcumin and bicalutamide enhanced the growth inhibition of androgen-independent prostate cancer cells through SAPK/JNK and MEK/ERK1/2-mediated targeting NF-κB/p65 and MUC1-C. Journal of Experimental & Clinical Cancer Research, 34 (1), 1–11. https://doi.org/10.1186/s13046-015-0168-z
Mirzaei, H., Shakeri, A., Rashidi, B., Jalili, A., Banikazemi, Z., & Sahebkar, A. (2017). Phytosomal curcumin: A review of pharmacokinetic, experimental, and clinical studies. Biomedicine & Pharmacotherapy, 85 (1), 102–112. https://doi.org/10.1016/j.biopha.2016.11.098
Mohebbati, R., Anaeigoudari, A., & Khazdair, M. (2017). The effects of Curcuma longa and curcumin on reproductive systems. Endocrine Regulations, 51 (4), 220–228. https://doi.org/10.1515/enr-2017-0024
National Toxicology Program (1993). NTP toxicology and carcinogenesis studies of turmeric oleoresin (CAS No 8024-37-1) (major component 79%-85% curcumin, CAS No 458-37-7) in F344/N rats and B6C3F1 mice (feed studies). National Toxicology Program Technical Report Series, 427 (1), 1–275.
Nejati-Koshki, K., Akbarzadeh, A., & Pourhassan-Moghaddam, M. (2014). Curcumin inhibits leptin gene expression and secretion in breast cancer cells by estrogen receptors. Cancer Cell International, 14 (1), 1–7. https://doi.org/10.1186/1475-2867-14-66
Piccolella, M., Crippa, V., Messi, E., Tetel, M. J., & Poletti, A. (2014). Modulators of estrogen receptor inhibit proliferation and migration of prostate cancer cells. Pharmacological Research, 79 (1), 13–20. https://doi.org/10.1016/j.phrs.2013.10.002
Ravindran, J., Prasad, S., & Aggarwal, B. B. (2009). Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? The AAPS Journal, 11 (3), 495–510. https://doi.org/10.1208/s12248-009-9128-x
Richter, E., Srivastava, S., & Dobi, A. (2007). Androgen receptor and prostate cancer. Prostate Cancer and Prostatic Diseases, 10 (2), 114–118. https://doi.org/10.1038/sj.pcan.4500936
Sahoo, D. K., Roy, A., & Chainy, G. B. (2008). Protective effects of vitamin E and curcumin on L-thyroxine-induced rat testicular oxidative stress. Chemical Biology Interactions, 176 (2–3), 121–128. https://doi.org/10.1016/j.cbi.2008.07.009
Sharma, R. A., Euden, S. A., Platton, S. L., Cooke, D. N., Shafayat, A., Hewitt, H. R., et al. (2004). Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clinical Cancer Research, 10 (20), 6847–6854. https://doi.org/10.1158/1078-0432.CCR-04-0744
Sharma, R., Gescher, A., & Steward, W. (2005). Curcumin: The story so far. European Journal of Cancer, 41 (13), 1955–1968. https://doi.org/10.1016/j.ejca.2005.05.009
Sharma, V., Kumar, L., Mohanty, S. K., Maikhuri, J. P., Rajender, S., & Gupta, G. (2016). Sensitization of androgen-refractory prostate cancer cells to anti-androgens through re-expression of epigenetically repressed androgen receptor–synergistic action of quercetin and curcumin. Molecular and Cellular Endocrinology, 431 (1), 12–23. https://doi.org/10.1016/j.mce.2016.04.024
Shehzad, A., Qureshi, M., Anwar, M. N., & Lee, Y. S. (2017). Multifunctional curcumin mediates multitherapeutic effects. Journal of Food Science, 82 (9), 2006–2015. https://doi.org/10.1111/1750-3841.13793
Sikora, E., Bielak-Żmijewska, A., Magalska, A., Piwocka, K., Mosieniak, G., Kalinowska, M., et al. (2006). Curcumin induces caspase-3-dependent apoptotic pathway but inhibits DNA fragmentation factor 40/caspase-activated DNase endonuclease in human Jurkat cells. Molecular Cancer Therapeutics, 5 (4), 927–934. https://doi.org/10.1158/1535-7163.MCT-05-0360
Singh, S., & Aggarwal, B. B. (1995). Activation of transcription factor NF-κB is suppressed by curcumin (diferuloylmethane). Journal of Biological Chemistry, 270 (42), 24995–25000. https://doi.org/10.1074/jbc.270.42.24995
Song, X., Zhang, M., Dai, E., & Luo, Y. (2019). Molecular targets of curcumin in breast cancer. Molecular Medicine Reports, 19 (1), 23–29. https://doi.org/10.3892/mmr.2018.9665
Soni, K., & Kutian, R. (1992). Effect of oral curcumin administration on serum peroxides and cholesterol levels in human volunteers. Indian Journal of Physiology & Pharmacology, 36 (4), 273–275.
Strimpakos, A. S., & Sharma, R. A. (2008). Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxidants & Redox Signaling, 10 (3), 511–546. https://doi.org/10.1089/ars.2007.1769
Sørlie, T., Perou, C. M., Tibshirani, R., Aas, T., Geisler, S., Johnsen, H., et al. (2001). Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proceedings of the National Academy of Sciences, 98 (19), 10869–10874. https://doi.org/10.1073/pnas.191367098
Tsui, K. H., Feng, T. H., Lin, C. M., Chang, P. L., & Juang, H. H. (2008). Curcumin blocks the activation of androgen and interleukin-6 on prostate-specific antigen expression in human prostatic carcinoma cells. Journal of Andrology, 29 (6), 661–668. https://doi.org/10.2164/jandrol.108.004911
Verma, S. P., Salamone, E., & Goldin, B. (1997). Curcumin and genistein, plant natural products, show synergistic inhibitory effects on the growth of human breast cancer MCF-7 cells induced by estrogenic pesticides. Biochemical and Biophysical Research Communications, 233 (3), 692–696. https://doi.org/10.1006/bbrc.1997.6527
Wahlström, B., & Blennow, G. (1978). A study on the fate of curcumin in the rat. Acta Pharmacologica et Toxicologica, 43 (2), 86–92. https://doi.org/10.1111/j.1600-0773.1978.tb02240.x
Wang, R., Sun, Y., Li, L., Niu, Y., Lin, W., Lin, C., et al. (2017). Preclinical study using Malat1 small interfering RNA or androgen receptor splicing variant 7 degradation enhancer ASC-J9® to suppress enzalutamide-resistant prostate cancer progression. European Urology, 72 (5), 835–844. https://doi.org/10.1016/j.eururo.2017.04.005
ahebkar, A. (2014). Curcuminoids for the management of hypertriglyceridaemia. Nature Reviews Cardiology, 11 (2), 123. https://doi.org/10.1038/nrcardio.2013.140-c1
alsalam, S., Agastian, P., Arasu, M. V., Al-Dhabi, N. A., Ghilan, A.-K. M., Kaviyarasu, K., et al. (2018). Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in vitro antibacterial, antifungal, antioxidant, and anticancer properties. Journal of Photochemistry and Photobiology B: Biology. https://doi.org/10.1016/j.jphotobiol.2018.12.010
alsalam, S., Agastian, P., Esmail, G. A., Ghilan, A.-K. M., Al-Dhabi, N. A., & Arasu, M. V. (2019). Biosynthesis of silver and gold nanoparticles using Musa acuminata colla flower and its pharmaceutical activity against bacteria and anticancer efficacy. Journal of Photochemistry and Photobiology B: Biology, 201, 111670. https://doi.org/10.1016/j.jphotobiol.2019.111670
anerjee, S., Singh, S. K., Chowdhury, I., Lillard Jr., J. W., & Singh, R. (2017). Combinatorial effect of curcumin with docetaxel modulates apoptotic and cell survival molecules in prostate cancer. Frontiers in Bioscience, 9, 235. https://doi.org/10.2741/e798
ggarwal, B. B., Surh, Y.-J., & Shishodia, S. (2007). The molecular targets and therapeutic uses of curcumin in health and disease. Springer Science Business Media, 1 (1), 1. https://doi.org/10.1007/978-0-387-46401-5
iftci, O., Tanyildizi, S., & Godekmerdan, A. (2010). Protective effect of curcumin on immune system and body weight gain on rats intoxicated with 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin (TCDD). Immunopharmacology and Immunotoxicology, 32 (1), 99–104. https://doi.org/10.3109/08923970903164318
illian, P. H., Kronski, E., Michalik, K. M., Barbieri, O., Astigiano, S., & Sommerhoff, C. P. (2012). Curcumin inhibits prostate cancer metastasis in vivo by targeting the inflammatory cytokines CXCL1 and-2. Carcinogenesis, 33 (12), 2507–2519. https://doi.org/10.1093/carcin/bgs312
iwari, A. K., Sodani, K., Dai, C.-L., Ashby, C. R., & Chen, Z.-S. (2011). Revisiting the ABCs of multidrug resistance in cancer chemotherapy. Current Pharmaceutical Biotechnology, 12 (4), 570–594. https://doi.org/10.2174/138920111795164048
ragg, G. M., & Newman, D. J. (2005). Plants as a source of anti-cancer agents. Journal of Ethnopharmacology, 100 (1–2), 72–79. https://doi.org/10.1016/j.jep.2005.05.011
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2025. All Rights Reserved.