
Kinetics and Mechanism of Electron-Transfer Reaction: Oxidation of n-butanaldehyde by N-chloroisonicotinamide in Aqueous Acetic Acid Medium
Birendra Singh 1, Umesh Kumar Vishwakarma 1
1 Department of Chemistry, S.G.S. Govt. P.G. College, Sidhi-486661, (M.P.), India
|
|
ABSTRACT |
||
|
N-chloroisonicotinamide uncatalyzed oxidation of butanaldehyde in aqueous acetic acid, and sulfuric acid
medium was investigated at 313 K. Data indicate the
reaction follows identical kinetics with first-order
in [oxidant]. The reaction rate shows direct proportionality with respect to
low [substrate], which tends to become zero-order at higher concentrations of
the butanaldehyde, and inverse fractional-order in
[H+] that follows kobs = a + b [H+]. The rate
increased with decreasing dielectric constant of the medium. The variation of
ionic strength, and the addition of reaction product (isonicotinamide)
had no significant effect on reaction rate. The stoichiometric ratio was
assigned 1:1 in H2O+Cl reacting species of oxidant mechanism with rupturing
C-H bond of substrate to yield n-butyric acid product. The activation
parameters associated with the rate-determining step have been computed. The
proposed mechanism, and the derived rate law are consistent with the observed
kinetic data. |
|||
|
Received 05 January 2025 Accepted 06 February 2025 Published 13 March 2025 DOI 10.29121/granthaalayah.v13.i2.2025.5991 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2025 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: n-butanaldehyde,
N-chloroisonicotinamide, Stoichiometry, Kinetics,
Oxidation |
|||
1. INTRODUCTION
The chemistry of N-haloisonicotinamide Priya & Subalakshmi (2018) has been of much importance both in analytical as well as pharmaceutical field. This readily available reagent offers trouble free work up of the reaction product compared to the other oxidants being as a main source of halogenium cation, hypohalite species, and nitrogen an ions behaves due as basses, and nucleophiles. Priya & Subalakshmi (2019), Pushpalatha (2015), Alhaji et al. (2011), Kol et al. (2019) This interesting property of NCIN, particularly as an oxidant, and chlorinating reagents have used in antimicrobial activities. n-butanaldehyde considered to be an important industrial usage Puttaswami et al. (2000) in perfumery resin (solvents) and agrochemicals. Its main source are incomplete combustion of fuels, wood smoke and biomass burning plumes. The paper relate to reactivity indifferent medium have been the subject matter of its redox process of kinetics. n-butanaldehyde contains carbonyl group (>C=O), the interaction between aldehyde, and H+ ions, Kemp (1972) and NCIN appears to be an interesting study as exhibits tautomeric phenomenon i.e. whether enol / carbonyl Bell (1968) form or gem-diol involve in the kinetics. Although very few kinetics of aliphatic aldehydes are reported in the literature with variety of reagents such as bromine, Aandam & Gopalan (1979) chloramine-T, Manadevappa et al. (1981) tetramethylammonium bromide, Asghar et al. (2019) Cr(VI) compounds, Panwar et al. (2013), Sharma et al. (2021), Kumar et al. (2011), Chaurasia, & Tiwari (2023) etc. It is therefore of interest to investigate the kinetics of butanaldehyde reaction with oxidizing agent NCIN, and ascertained NCIN to the logical information regarding the elucidation of mechanism.
2. EXPERIMENTAL
N-chloroisonicotinamide was synthesized by the reported method. Priya & Mathiyalagan (2011) The purity of NCIN was checked iodometrically and through IR spectrum. The afresh solution of NCIN was prepared in sufficient quantity of acetic acid (B.D.H.): water (80:20%, v/v) mixture, standardized by iodometric method, and preserved in brown bottle, kept in dark at refrigerated temperature (~50C) to save from the occurrence of photochemical reactions, and to enhance stability considerably.
n-butanaldehyde (B.D.H.) was used after purification. Agrawal et al. (1990), Perrin et al. (1966) A solution of compound was prepared in demineralized water, and acetic acid mixture. All other solutions either of chemicals, AnalaR or GR quality were used as supplied.
3. Kinetic Determinations
In kinetic runs, butanaldehyde
concentration was maintained in excess over the [NCIN]. Rate values (![]() ),
obtained from the initial slope of individual graphs between the residual
concentrations of NCIN at various time intervals, were finally plotted against
time, the changing concentrations of the reactant for which order of the
reaction was to be determined. Order with respect to NCIN was confirmed by
plotting
),
obtained from the initial slope of individual graphs between the residual
concentrations of NCIN at various time intervals, were finally plotted against
time, the changing concentrations of the reactant for which order of the
reaction was to be determined. Order with respect to NCIN was confirmed by
plotting ![]() values versus time (oxidant variation).
values versus time (oxidant variation).
The reaction was initiated in glass-stoppered Pyrex vessel whose outer surface was coated black to eliminate photochemical effect. Requisite amount of solutions of butanaldehyde, acid, water, were taken in the flask and thermostated at 313 K for thermal equilibrium.
A measured quantity of the NCIN (oxidant) solution also thermostated at the same temperature, was rapidly added to the mixture with constant stirring in the flask. The progress of the reaction was monitored by iodometric determination of unreacted NCIN in measured aliquots of the rection mixture withdrawn at different intervals of time. The pseudo first-order rate constant (k) calculated were reproducible (± 4%).
4. Results
The stoichiometry of the reaction was measured by taking a known excess of [NCIN] over [butanaldehyde]. The stoichiometry observed was 1:1 and it could be represented as :
![]()
n-butyric acid as product was detected, and established in the oxidation of n-butanaldehyde by forming its amide (m.p. = 1150C), and also chemical method. Vogel (2010)
The oxidation kinetics of substrate was carried out under pseudo first-order conditions, with [n-butanaldehyde] has been higher than, atleast to ten times that of [NCIN] (Table 1). The examination of the results indicate that the rate is first-order with respect to oxidant. Plots of log [NCIN]0 / [NCIN]t versus time (Fig.1) were linear/ parallelism even up to 75% to 80% conversion of [NCIN]. The slope values of kobs were independent of [NCIN].
Table 1
|
Table 1 Effect of Variation of [NCIN] on Rate at 400C [butanaldehyde] = 5.00 ×
10-3 mol dm-3 ; [H+]
= 1.00 mol dm-3 ; CH3COOH-H2O
= 75 %, (v/v) |
||
|
S.No. |
[NCIN] × 10-3 (mol dm-3) |
104 k (s-1) |
|
1. |
1.25 |
2.69 |
|
2. |
2.00 |
2.72 |
|
3. |
2.50 |
2.67 |
|
4. |
3.33 |
2.75 |
|
5. |
4.00 |
2.68 |
|
6. |
5.00 |
2.64 |
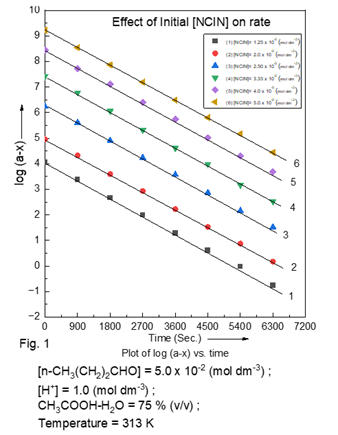
5. Butanaldehyde Concentration Dependence
The concentration of benzaldehyde was varied from 1.50 ×10-2
to 6.25 10-2 mol dm-3 keeping constant concentration of
other ingredients at 313 K. The rate of oxidation was observed to increase, and then decrease by raising the concentration of
substrate showing order one to zero. The validity of Michaelis-Menten type
kinetics was established by data. The existence of complex formed in
preequilibrium state at transition state was graphically proved by plotting
double reciprocal plot (![]() against
against ![]() )
with positive intercept on Y-axis.
)
with positive intercept on Y-axis.
The effect of increasing concentration of hydrogen-ion indicate that oxidation rate slowly accelerates, and follows first-order kinetics in [H+] but attains a limiting value of rate constant at its higher concentration has the form kobs = a + b [H+]. The order of reaction derived from the slope of plot of log k vs. log [H+] was found less than unity. The rate increases with decrease in dielectric constant in this case, the results suggest that the reaction may take place between ions of opposite charge. The addition of reaction mixture to aqueous acrylonitrile/acryloamide/BHT solution did not initiate polymerisation showing the absence of free radical species, rules out the participation in reaction mechanism.
The variation of the ionic strength of the medium, sodium perchlorate, or addition of the reaction product isonicotinamide had negligible effect on the reaction rate.
6. Discussion
SenGupta et al. (1982) have shown the existence of similar equilibria in acid solution of bromine like its N-chloroisonicotinamide behaves a strong electrolyte in aqueous solution forming different species as shown in equations (2-4).
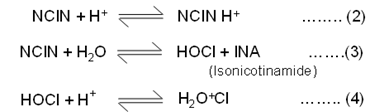
In aqueous acetic acid, the probable oxidizing species is H2O+Cl.
It is believed that reaction proceed through the formation, and subsequent decomposition of complex. Under basic conditions, however, the electron is accelerated by electron withdrawing groups, and is supposed to be a free-radical chain process or to involve hydride transfer. Freeman et al. (1970) The hydrates of aliphatic aldehydes are well known, Greenzaid et al. (1967) and the oxidations of some aliphatic aldehydes have been shown to occur through intermediate hydrates.
7. Mechanism
Considering oxidizing species ![]() of oxidant, and hydrated form of butanaldehyde
(organic substrate) a plausible reaction mechanism can be envisaged as follows:
of oxidant, and hydrated form of butanaldehyde
(organic substrate) a plausible reaction mechanism can be envisaged as follows:
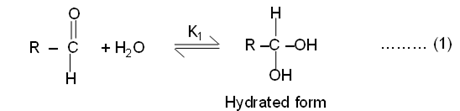
where, R = CH3 CH2CH2- for n-butanaldehyde.
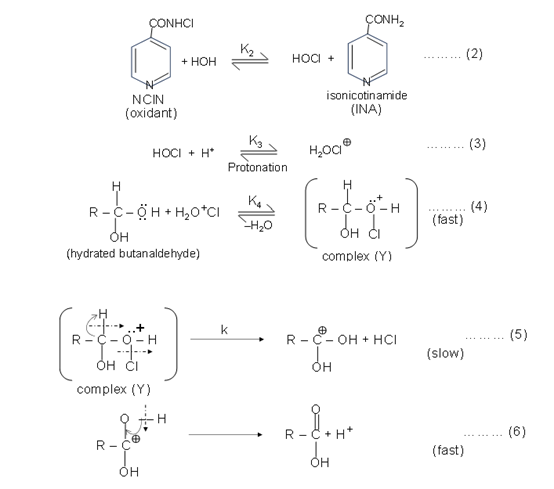
Here, the complex (Y) is defined as above.
In view of above mechanistic paths, the disappearance of NCIN, which leads to the following rate law:
![]()
![]()
or ![]()
Substituting the concentration of [NCIN]t, and simplification, results rate law as:
![]()
On execution of steady-state approach, the kobs takes the form
![]()
Further the double reciprocal form of equation (11) found linear with non-zero intercept leads equation (12).
![]()
This tentatively account for the events of mode of electron transfer between oxidant, and substrate in the reaction, and explains rate was fraction in [butanaldehyde]. Michaelis-Menten type kinetics was verified. It also explains al the kinetic results Gem-diol species with positive carbon being most unstable looses a proton by C-H bond fission, and changes to the final product i.e. carboxylic acid. The proposed mechanism is realistics in justifying for many of the observed kinetic data using the calculated ‘k’ values. The activation parameters (Table 2) were determined, and plots of log10 k vs. 1/T (Fig.2) were made.
Table 2
|
Table 2 Variation of Temperature on Rate [NCIN] = 5.00 × 10-3 mol dm-3; [H+] = 1.00 mol
dm-3 ; CH3COOH-H2O
= 75 %, (v/v) |
|||||
|
S. No. |
102 ×[butanaldehyde] (mol dm-3) |
104× k (s-1) |
|||
|
Temp. (T) K |
308 |
313 |
318 |
323 |
|
|
1. |
3.33 |
1.51 |
2.13 |
3.01 |
4.25 |
|
2. |
5.00 |
1.87 |
2.64 |
3.73 |
5.25 |
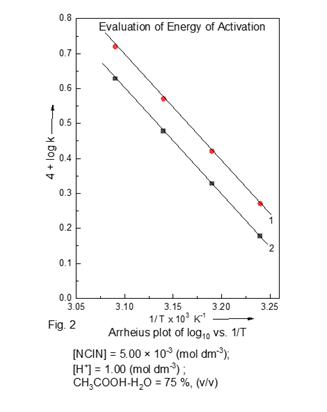
Ea : 57.46 kJ mol-1,
ΔH# : 55.02 kJ mol-1,
ΔG# : 90.75 kJ mol-1,
-ΔS# : 113.24 JK-1 mol-1,
Temperature coefficient : 1.41, 1.99 for 50 C, and 100 C respectively.
The large negative value of entropy of activation (ΔS#) accounts for an orientation of transition state, and solvation of the activated complex. The size of the substate is significantly large, hence, more -ΔS# value can be assigned to it as suggested earlier. Edwards (1964)
8. Conclusion
The interaction between gem-diol form of butanaldehyde, and protonated species H2O+Cl of the oxidant NCIN in aqueous acetic acid medium is a fractional-order kinetics. The reaction follows kobs = a + b [H+] in case of [H+] effect. A stoichiometrically 1:1 mechanism based on an adduct formation has been considered to account for the Michaelis-Menten type kinetics. The study observed the rupture of C-H bond in the rate-determining step.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
Our thanks to Principal and Head, Department of Chemistry, Assistant Professor, Umesh Kumar Vishwakarma, Department of Chemistry, S.G.S. Govt. P.G. College, Sidhi (M.P.) for providing laboratory work, and helpful discussions.
REFERENCES
Aandam, S., & Gopalan, R. (1979). Indian Journal of Chemistry Section A, 17A(6), 629.
Agrawal, A., Choudhary, K., & Banerji, K. K. (1990). Journal of Chemical Research, 5, 86-87.
Alhaji, N. M. I., Uduman Mohideen, A. M., & Kalamathi. (2011). E-Journal of Chemistry, 8(1), 1-8.
Asghar, B. H., Malik, S., & Mansoor Sheikh, S. (2019). Arabian Journal of Chemistry, 12, 1252-1259. https://doi.org/10.1016/j.arabjc.2014.10.047
Bell, R. P. (1968). Advances in Physical Organic Chemistry, 4, 1.
Chaurasia, S. K., & Tiwari, S. (2023). International Journal of Science Development and Research, 8(7), 867-871.
Edwards, J. O. (1964). Inter Science. New York: Wiley.
Freeman, F., Brant, J. B., Heser, N. B., Kamego, A. A., Kasner, M. L., McLaughlin, T. G., & Paull, E. W. (1970). Journal of Organic Chemistry, 35, 982. https://doi.org/10.1021/jo00829a025
Greenzaid, P., Rappoport, Z., & Samuel, D. (1967). Transactions of the Faraday Society, 63, 2131. https://doi.org/10.1039/tf9676302131
Kemp, J. J. (1972). In Bamford, O. C. H. & Tipper, C. F. H. (Eds.), Comprehensive Chemical Kinetics (Vol. 7, p. 4). Elsevier, New York.
Kol, S., Singh, S. K., Sharma, K. N., Verma, B., & Suryavanshi, S. (2019). E-Journal of Advanced Research, 5(1), 35-44. https://doi.org/10.1016/j.jare.2018.12.005
Kumar, P., Pandey, D., & Kothari, S. (2011). Croatica Chemica Acta, 84(1), 6212-6224.
Manadevappa, D. S., Jadhav, M. B., & Naidu, H. M. K. (1981). Journal of the Indian Chemical Society, 58, 454.
Panwar, S., Pohani, S., Swami, P., Vyas, S., & Sharma, P. K. (2013). European Chemical Bulletin, 2(11), 904-909.
Perrin, D. D., Perrin, D. R., & Armarego, W. L. (1966). Purification of Organic Compounds. Oxford: Pergamon Press.
Priya, V., & Mathiyalagan, N. (2011). Asian Journal of Chemistry, 623(4), 1871-1872.
Priya, V., & Subalakshmi, M. (2019). International
Journal of Innovative Science, Engineering, and Technology, 6, 12. https://doi.org/10.31788/RJC.2019.1235213
Priya, V., & Subalakshmi, M. (2018). International Journal of Research in Applied Science and Engineering Technology, 61(1), 2099-2103. https://doi.org/10.22214/ijraset.2018.1330
Pushpalatha, L. (2015). International Letters of Chemistry, Physics, and Astronomy, 52, 111-119. https://doi.org/10.56431/p-np5y9f
Puttaswami, M., Anuradha, T. M., Ramachandrappa, R., & Made Gowda, N. M. (2000). International Journal of Chemical Kinetics, 32, 221. https://doi.org/10.1002/(SICI)1097-4601(2000)32:4<221::AID-KIN4>3.3.CO;2-T
SenGupta, K., Samadar, H. P., Sen, P. K., & Banerjee, A. (1982). Journal of the American Chemical Society, 82, 3022-3263.
Sharma, J., Singadiya, A., Prakash, O. M., & Sharma, V. M. F. C. (2021). Journal of Emerging Technologies and Innovative Research, 8(4), 167-173.
Vogel, A. I. (2010). Elementary Practical Organic Chemistry.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2025. All Rights Reserved.