
Kinetics of Oxidation of Unsaturated Acid by Thallium(III) in Aqueous Acetic Acid and Micellar Medium
Jafar Jilane 1, K.N. Sharma 2
1 Department
of Chemistry, A. P. S. University, Rewa (M.P.) India
2 Department of Chemistry, S.G.S. Govt. P.G. College, Sidhi-486661, (M.P.), India
|
|
ABSTRACT |
||
|
The oxidation
of α-crotonic acid by thallium (III)
perchlorate proceeds by a mechanism approach involving direct two-electron
transfer with lack of free radical intervention from substrate to the oxidant
via an intermediate complex. The stoichiometry (1:1) of the reaction followed
by its decomposition in a rate-determining step to product
acetaldehyde. The orders in oxidant and acid were found to be unity, whereas
solvent, micellar (CTAB) exhibited fraction-order kinetics. The activation
parameters were also discussed. |
|||
|
Received 02 January 2025 Accepted 05 February 2025 Published 13 March 2025 DOI 10.29121/granthaalayah.v13.i2.2025.5990 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2025 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Crotonic Acid,
Thallium (III), Perchlorate, Cetyltrimethylammonium Bromide (CTAB),
Oxidation, Stoichiometry |
|||
1. INTRODUCTION
The chemistry of α-crotonic acid, an unsaturated acid containing a double bond, and carboxylic a functional group in non-aqueous solvent causes cleavage of >C=C bond. Owing to this properly, it received much attention in the recent past due to its applications in biochemistry Banerji (1972) solvolysis process, synthetic material chemistry and pharmaceuticals. α-crotonic acid behaves differently than other aliphatic, and aromatic carboxylic acids towards many oxidants. Oxidation of crotonic acid by chromine-T, Shrivastava & Neelam (2015) N-bromoisonicotinamide Prajapati et al. (2019) and N-chlorosaccharin Singh, & Dubey (2023) etc. have been reported. The micelles are of special significance because of their biological relevance, and are also important in enzyme Josh et al. (2006), Agnihotri & Tiwari (2021), Singh & Swami (2022) catalysed (CTAB) reactions as well as a solvent. Bhuvaneshwari & Elango (2006), Ramachandrappaet al. (1998) The thallium(III) perchlorate is used as oxidant for various organic transformations Hemkar et al. (2013) Tunable physical properties like redox potential, and acidity of transition metal makes it attraction from catalytic point of view. Most of the electron transfer reactions of Tl(III), and their mechanistic studies concentrated. The oxidation of crotonic acid by Tl+3 was undertaken to elaborate the mechanism of the reaction prone to form complex with Tl+3 in aqueous acetic acid, and micellar medium for the tittle reaction.
2. EXPERIMENTAL
Materials: All the solutions of the reagents were prepared in double distilled water. The micellar cationic cetyltrimethylammonium bromide (Sigma), and α-crotonic acid (S.D. fine) were used as such received without purification. The solution of sodium thiosulphate was freshly prepared every day, and standardized iodometrically. Thallium(III) perchlorate oxidant was prepared by reported method Shah et al. (2022) as follows. The sample of thallic oxide (B.D.H.) in calculated amount was dissolved in 70% perchloric acid (AREM), and the solution was heated to 110°C for about 15 minutes with constant stirring, quickly filtered and cooled. The solution of Tl(III) perchlorate was standardized by iodometric method.
Kinetic
measurements
The reaction between Tl+3, and crotonic acid was studied under pseudo first-order conditions [crotonic acid] >> [Tl+3] at constant temperature of ± 0.1°K. The reaction was initiated by mixing previously thermostated solutions of substrate, and oxidant which also contained the required amount of HClO4, CH3COOH, and micellar cationic CTAB. The reaction was followed upto ca. 80% conversion by monitoring the concentration of remaining Tl+3 iodometrically. The pseudo first-order rate constants were determined from the plots of log [Tl+3] against time, and the rate constants were reproducible to within ±3%.
Test for free
radicals
No precipitate due to the polymerization of the added
acrylonitrile / acryloamide (monomer) was observed,
thus discorded the presence of any free radicals present in the reaction. Mahesh et al. (2004)
3. Results and Discussion
Stoichiometry and
Products analysis
The stoichiometric of the reaction was determined under kinetic conditions when crotonic acid concentration was taken sufficiently lower than that of Tl+3 in acetic acid, perchloric acid, and micellar medium. The excess of [Tl+3] was estimated iodometrically, and the results indicated a reaction of a mole of crotonic acid for a mole of per Tl+3 as represented in equation .
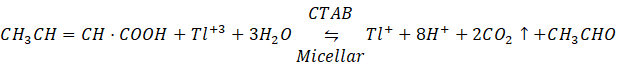 …. (1)
…. (1)
The reaction mixture containing excess of thallium(III) perchlorate was kept undisturbed upto 24 h, and the complete disappearance of the crotonic acid was confirmed chromatographically. The
aldehyde product was isolated from the reaction mixture by forming its 2:4-DNP
derivative characterized by its m.p. determination
(162°C). The reaction is of first-order
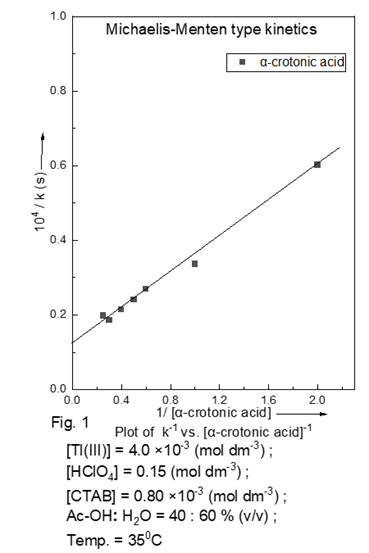
with respect to Tl+3. Michaelis-Menen type kinetics is observed with
respect to the crotonic acid (Table 1), as verified by double reciprocal plot of ![]() vs. [substrate] -1 (Fig. 1).
vs. [substrate] -1 (Fig. 1).
Table 1
|
Table 1 Dependence of kobs on [α-crotonic acid] at 35°C [Tl(III)] = 4.0 ×10-3
(mol dm-3) ; [HClO4]
= 0.15 (mol dm-3) ; [CTAB] =
0.80 ×10-3 (mol dm-3) ; CH3COOH:H2O = 40 :60
%, (v/v) |
||
|
[α-crotonic
acid] ×10-2 (mol dm-3) |
kobs 104
s-1 |
|
|
0.50 |
1.66 |
3.32 |
|
1.00 |
2.97 |
2.97 |
|
1.66 |
3.70 |
2.22 |
|
2.00 |
4.15 |
2.07 |
|
2.50 |
4.64 |
1.85 |
|
3.33 |
5.36 |
1.60 |
|
4.00 |
5.05 |
1.26 |
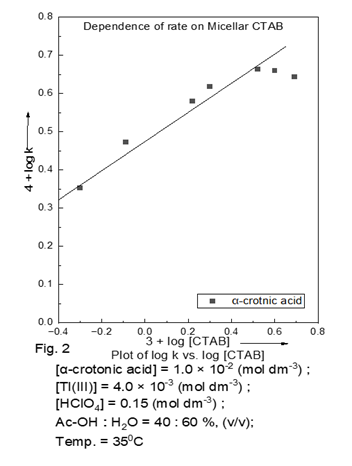
The reaction is catalysed by H+ ions. The oxidation of α-crotonic acid was studied in binary organic solvents viz. acetic acid, and micellar (CTAB). CTAB attributed to capability of forming intermediate complex (Table 2), but the rate constant exhibited much variation in values with micellar solvent plot of log kobs vs. log [CTAB] (Fig. 2). The data on the solvent effect showed an excellent correlation in terms of Swain’s equation. Swain (1983) The successive addition of neutral salt, and ionic strength of the medium have no effect on rate.
Table 2
|
Table 2 Dependence of kobsd on micellar [CTAB] at 35°C [α-crotonic acid] = 1.0
×10-2 (mol dm-3) ; [Tl(III)] = 4.0 ×10-3 (mol dm-3)
; [HClO4] = 0.15 (mol dm-3) ; AcOH:H2O = 40 : 60 %,
(v/v) |
||
|
103 × [CTAB] (mol dm-3) |
kobs 104
s-1 |
|
|
0.50 |
2.25 |
0.45 |
|
0.80 |
2.97 |
0.37 |
|
1.66 |
3.81 |
0.22 |
|
2.00 |
4.15 |
0.20 |
|
3.33 |
4.62 |
0.13 |
|
4.00 |
4.57 |
0.11 |
|
5.00 |
4.41 |
0.08 |
4. Rection mechanism
Since a one electron oxidation giving rise to free radicals is unlikely. The reaction proceeds with the rapid formation of complex between substrate, micelles and Tl+3. The subsequent rate-determining decomposition of complex to yield acetaldehyde confirming the cleavage of the C-C bond in slow step. This leads to the postulation of following þath ways of mechanism, and rate law.
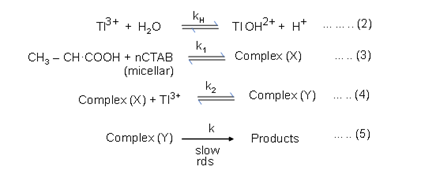
Execution of stead-state approach, the rate is expedited as :
kobs = ![]() ........ (6)
........ (6)
The rate expression explains all observed kinetic facts under different conditions.
The moderate values of ΔE# = 57.87 kJ mol-1,
ΔH# = 54.72 kJ mol-1, ΔG# = 84.24
kJ mol-1, and ΔS#
= -95.01 JK-1 mol-1 were favourable for electron transfer
processes. The negative value of ΔS#, can be ascribed to the
nature of electron pairing and unpairing processes and to the loss of degree of
freedom formerly available to reactant upon the formation of transition state. Lene et al. (2005)
5. Conclusion
The reaction followed Michaelis-Menten type kinetics both respect to α-crotonic acid. The order with respect to oxidant Tl+3 is unity. Micelles cationic CTAB acts both as catalyst and solvent. The stoichiometric ratio was found 1:1. The derived rate law based on proposed mechanism explains all the kinetic facts.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
The author is very thankful to Professor and Head, Department of Chemistry, A. P. S. University, Rewa (M.P.) for providing facilities and guidance.
REFERENCES
Agnihotri, M., & Tiwari, S. (2021). Int. J. Theor. & Appl. Sciences, 13(2), 12-16.
Banerji, K. K. (1972). Journal of the Indian Chemical Society, 49, 1097.
Bhuvaneshwari, D. S., & Elango, K. P. (2006). International Journal of Chemical Kinetics, 38, 657-665. https://doi.org/10.1002/kin.20199
Hemkar, S., Riya, S., Khandelwal, C., & Sharma, P. D. (2013). American Journal of Physical Chemistry, 2(5), 73-79.
Josh, G. K., Katre, Y. R., & Singh, A. K. (2006). Journal of Surfactants and Detergents, 9, 231-235. https://doi.org/10.1007/s11743-006-5002-3
Lene, G., Fabian, I., & Poe, A. I. (2005). New Journal of Chemistry, 29, 759-760.
Mahesh, R. T., Belakki, M. B., & Nandibewoor, S. T. (2004). Catalysis Letters, 97, 91-98. https://doi.org/10.1023/B:CATL.0000034293.78427.8d
Prajapati, A., Dwivedi, A. P., & Parihar, S. S. (2019). International Journal of Chemistry Science, 3(4), 9-11.
Ramachandrappa, R., Puttaswamy, Mayanna, S. M., & Made Gowda, N. B. (1998). International Journal of Chemical Kinetics, 30(6), 407-414. https://doi.org/10.1002/(SICI)1097-4601(1998)30:6<407::AD-KIN2>3.0.CO;2-W
Shah, N. H., Dar, N. A., Farhad, & Singh, S. P. (2022). Chemical Society Transactions, 11(2-4), 26-32.
Shrivastava, A., & Neelam. (2015). International Journal of Applied Research, 10, 380-384.
Singh, N., & Swami, M. N. (2022). International Journal of Theoretical & Applied Sciences, 14(91), 53-56.
Singh, R., & Dubey, V. (2023). International Journal of Science Development and Research, 8(1), 1036-1039.
Swain, C. G., Swain, M. S., Powel, A. L., & Alunni, S. (1983). Journal of the American Chemical Society, 105, 502. https://doi.org/10.1021/ja00341a033
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2025. All Rights Reserved.