
Fourier Transform Infrared Spectroscopic (FT-IR) study of Dicofol-Induced Structural and Biochemical Perturbations on Rattus norvegicus Testis
1 PhD.,
Student, Environmental Toxicology and Molecular Biology Laboratory, Department
of PG Studies and Research in Zoology, Karnatak University, Dharwad- 580003
2 Professor,
Environmental Toxicology and Molecular Biology Laboratory, Department of PG
Studies and Research in Zoology, Karnatak University, Dharwad- 580003
|
|
ABSTRACT |
||
|
The toxicity
of organochlorine has drawn a lot of attention
recent times due to its widespread industrial use and reputation as a
widespread environmental pollutant. In order to
assess the organochlorine acaricide Dicofol's (DCF) toxic effects on the rat
reproductive system at the molecular level, the present study employed
histopathological investigations and the FT-IR technique. Rats were randomly
assigned to four groups C, D1, D2, and D3 for this purpose. For 90 days, each
group was given 00, 5, 7, and 10 mg/100g of body weight. All
of the FT-IR peaks and the histological analysis revealed a negligible
change in the group that received lower doses of D1. The area under the
peaks, which correspond to various biomolecules, significantly decreased in
the groups treated with higher doses of D2 and D3. Furthermore, when
comparing the testes of the D2 and D3 groups to the control group,
histopathological examination of seminiferous tubules at varying dosage
levels showed mild to severe degenerative changes. In summary, the higher
dosage of dicofol that was chosen resulted in considerable testicular damage,
which impacts male fertility. Consequently, the use of such an acaricide
ought to be restricted to a planned program. |
|||
|
Received 30 October
2024 Accepted 12 November 2024 Published 15 December 2024 Corresponding Author Dr. M David, mdavid.kud@gmail.com DOI 10.29121/granthaalayah.v12.i11.2024.5860 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2024 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Dicofol, FT-IR study, Male Wistar Rat,
Testis, Histology |
|||
1. INTRODUCTION
Pesticides are used for a variety of domestic and commercial purposes, including promoting agricultural production and safeguarding the health of people and animals (Köhler & Triebskorn, 2013, Tudi et al. (2021)). Pesticides are used to control pests and weeds as a function of their chemical ingredients, therefore, they can also be toxic to other organisms, including, fish, amphibians, beneficial insects, and non-target plants David et al. (2018), Kartheek & David (2018), Ramesh & David (2009), Tudi et al. (2021). Organochlorine insecticides (OCIs) are chemically stable xenobiotic compounds, belong to the class of persistent organic pollutants and have toxic effects on living organisms (Jayaraj et al. (2016), Zheng et al. (2020)). The organochlorine pesticide dicofol 2,2,2-trichloro-1,1-bis(4-chlorophenyl) ethanol shares a chemical relationship with DDT. It is used specifically as a miticide to combat the red spider mite (PubChem, n.d.). According to M. F. Ahmad et al., (2024) humans have been exposed to a variety of hazardous chemicals in the workplace and environment in recent years, particularly pesticides. According to the US EPA, (2013), dicofol can enter the human body through the mouth, skin, or lungs. Since dicofol shares a structure with DDT, it is viewed with similar concerns. This is because DDT has detrimental effects on both humans and animals, including bioaccumulation, long-range transport, persistence, endocrine disruption, and reproductive toxicity Ahmad & Ahmad (2017), Clark et al. (1990), El-Kashoury et al. (2010), Qi et al. (2022). The use of OCIs in public health and agriculture have not only uprooted the target pests, but it has also non targeted organisms on ecosystem Ben et al. (2021), Jayaraj et al. (2016). According to early research, there is growing evidence that exposure to OCIs can be harmful to both human and animal reproduction (Ben et al. (2021), Milesi et al., 2020). However, prior research has demonstrated that male rats exposed to DCF have significantly decreased rates of testicular and epididymal sperm as well as of serum testosterone El-Kashoury et al. (2010). Therefore, the objective of the current study was to investigate the subchronic effects of DCF-induced testicular damage in rats using molecular techniques (FT-IR).
2. Materials and Methods
2.1. Animals
Normal male Wistar albino rats weighing 180–190 grams were used in the experiment. Prior to the experiment, the animals were kept in holding facilities for two weeks at a temperature of 24 ± 2 °C, a relative humidity of 68 ± 5%, and a 12-hour light/dark cycle in accordance with the CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals) guidelines for the care and use of laboratory animals. A typical laboratory diet and unrestricted access to water were also provided to them.
2.2. Chemicals
Dicofol Difol Pesticide 18.5% (EC) was purchased from Pushpak Agro Services in Talegoan Dhabade, Pune, Maharashtra, India. The other chemicals used in this investigation were all commercially available and of analytical grade.
2.3. Experimental design
Following a week of acclimatization, the rats were split into four major groups of six animals each at random and given the following treatment.
C: Control (Only vehicle).
D1: 5 mg/100gms BW Dicofol (1/12th of LD50).
D2: 7 mg/100gms BW Dicofol (1/9th of LD50).
D3: 10 mg/100gms BW Dicofol (1/6th of LD50).
The selected LD50 (598 mg/ kg−1 BW Dicofol) value of DCF was based on the available literature (Vessela Vitcheva Dicofol 2011). The treatment was given orally with the dose volume of 1 ml 100 gm−1 BW in the morning time for 90 days. However, the first selected dose was considered to be relatively environmentally relevant (Ben et al. (2021), Fujii et al. (2011)). Therefore, in order to assess the toxicity on rat testis, we have chosen two additional doses with a higher sublethal concentration in accordance with OECD (Organization for Economic Cooperation and Development) guidelines for chemical testing on animals. At the end of 90days, rats from each of the four groups were anesthetized by chloroform and then euthanized. Testicular tissue samples were retrieved for the histopathological and FT-IR Studies.
2.4. Histopathology
The rat testicles were fixed in 10% neutral formalin, dehydrated in increasing alcohol grades, and embedded in paraffin wax in accordance with the Humason & Humason, (1962) method for histopathology. Using hematoxylin and eosin (H&E), paraffin sections that were 5 𝜇m thick were stained for a standard histological analysis.
2.5. Sample preparation and FT-IR spectral analysis
Rat testicles were prepared for FT-IR investigations using the procedure described by Shivanoor & David (2015), was followed in the preparation of the rat testis. Rat testicles were extracted after euthanasia and homogenized right away using liquid nitrogen. To eliminate the water content in the samples, they were subsequently dried for 12 hours in a lyophilizer (VIRTIS 6 KBEL 85). In an agate mortar and pestle, IR-grade potassium bromide (KBr) was used to grind thoroughly dried testis samples. A pellet press machine was used to make KBr pellets in triplets. The KBr pellets that were prepared had an 11 mm diameter and 1mm thickness. A Nicolet-6700 FT-IR spectrometer and an air-cold Dueterated Triglycine Sulfate detector were used to record FT-IR spectra in the range of about 4000–400 cm−1 at room temperature (26 ± 1°C). Each pellet was scanned in the same way, and the spectra were then further examined with the ORIGIN.8 program.
2.6. Statistical Analysis
Standard error of the mean (SEM) ± mean was used to express all data. P-values less than 0.05 were regarded as statistically significant in the Analysis of Variance (ANOVA) test, which was used to examine the group differences.
3. RESULTS AND DISCUSSIONS
3.1. Histological Studies
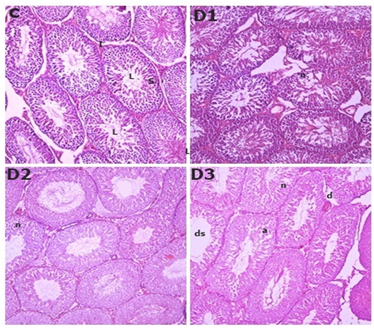
Rats exposed to 5, 7, and 10 mg/100 gm of DCF showed different testicular tissue in Figure 1compared to the control group. In the rats in the control groups, the histology of the seminiferous tubules and interstitial tissues showed normal structural characteristics for spermatogenic cells and sertoli cells. The D1 and D2 groups seminiferous tubule epithelium showed structural abnormalities such as necrosis after 90 days of DCF exposure, while the D3 group's seminiferous tubules of rat testis showed necrosis, atrophy, and depletion in spermatocytes with larger lumen space than the D1, D2, and Control groups Testicular abnormality increased dramatically in all treated groups in a dose-dependent manner Figure 1 Histological analyses of the treated animal's testes suggested that this happened as a result of toxic damage caused by dicofol to seminiferous tubules. It has been documented that test animals intoxicated with organochlorines exhibit testicular necrosis, atrophy, and degenerative epithelium of seminiferous tubules (Ahmad & Ahmad (2017), Ben et al. (2021), Cook et al., 2011). Our findings are consistent with those of Jadaramkunti & Kaliwal (2002), who found that treatment with DCF at a higher dose (500 mg/kg BW) for 30 days significantly decreased the weight of the testes and epididymus, as well as the total sperm count and the percentage of motile and live sperms compared to a lower dose 200mg/kg BW), for 30days dosing period. The findings of the current study concur with those of El-Kashoury et al. (2010). Dicofol appears to be more toxic at higher doses than at lower doses, according to the results obtained.
Figure1
|
Figure 1 H & E-stained testicular cross section of
control rats (C) showing normal regular seminiferous tubules having small
lumen (L) filled with spermatocytes, sertoli cells
(S) and normal Interstitial cells (I). D1 & D2 shows
necrosis in testicular section. D3 shows (ds) Depletion in spermatogonia and
spermatocyte cell forming large seminiferous lumen,
(d) Structural disruption in seminiferous epithelium, (a) Atrophy, (n)
Necrosis in seminiferous tubules after 90days of dicofol exposure. (C)×200,
(D1)×200, (D2)×200, (D3)×200. |
3.2. FT-IR studies
Figure 2
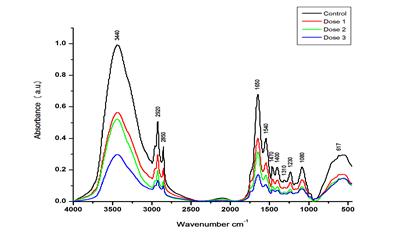
|
Figure 2 FT-IR Spectra of the Control and Various Doses of DCF-Treated Rat Testicles in the Range of Approximately 4000 to 500 Cm−1 |
In this study, the molecular abnormalities brought on by the DCF's toxic effect on rats testicles were examined using FT-IR spectroscopy. Figure 2 displays the control and various DCF dosages on rat testis FT-IR spectra (4000 – 500 cm−1). The FT-IR wave number and detailed band assignments were done according to (Mello & Vidal (2012), Talari et al. (2017)) Table 1. The stretching modes C-H, N-H, and O-H, Amide I peptide O=C-N-H stretching of α-helix, Amide-II (N-H bend, C-N stretch) of β-sheet, and Amide-III peptide CH2 stretching are represented by the FT-IR spectra bands centered at approximately ∼3440, ∼1650, ∼1540, and ∼1310 cm−1, respectively. The absorption in this range was dominated by the amid-A band at approximately 3445 cm−1 of protein, which is caused by the N-H and O-H stretching modes of proteins. When compared to the control group, the D1 group in the current study displayed negligible (P > 0.05) changes in the area under the ∼3445 cm−1 band Table 2. In contrast to the control group, the D2 and D3 groups displayed a significant (P < 0.05) decrease in the area value of these bands by −16% and −23%, respectively. The IR spectra of proteins in the range of ∼1700 to ∼1600 cm−1 were also examined in order to look into the changes in the secondary structure of proteins Figure 2. The total intensity of each peak in the protein secondary derivative in D2 and D3 varied significantly (P > 0.01) from the control. According to El-Kashoury et al. (2010), the testis lower protein level was indicated by the smaller area under these bands. The ratio of β-sheet to α-helix in the infrared band at approximately ∼1650 and ∼1540 cm−1 respectively showed that D2 and D3 had significantly more β-sheet than control.
The lipid content of rat testis was examined by considering the spectral range of approximately 3050 to 2800 cm−1 Figure 2. In this area, the bands are assigned, as shown in Table 1 Four prominent bands were identified in this spectral region at ∼2920, 2850, 1470, and 1400 cm−1. These bands were attributed to the stretching modes of CH2 asymmetric, CH2 symmetric, CH2 bending, and COO- symmetric stretching mode in lipids, respectively Table 1. Our data showed that, in comparison to the control, the area under these bands dropped insignificantly (P > 0.01) in the D1 group and significantly (P > 0.01) in the D2 and D3 groups. It has been investigated that a CH2 symmetric stretching band frequencies shifting to lower values indicates decreased membrane fluidity. (Liu et al., 1999), Jayaraj et al. (2016) and Pelletier et al. (2003) demonstrated that the reduction in lipids and cholesterol levels caused by exposure to organochlorines may be a sign of testicular damage.
Table 1
|
Table 1 FT-IR Spectra of Rats Exposed to Varying Concentrations of Commercial-Grade Dicofol in the 4000–500cm-1 Spectral Range, With General Band Assignments Obtained from the Literature (Mello & Vidal, 2012; Talari Et Al., 2017) |
|
|
Wavenumber cm−1 |
Band Assignment |
|
3440 |
X-H
asymmetric stretching vibrations (where X is C, O, or N) – Amide |
|
2920 |
CH2 asymmetric stretching: mainly lipids |
|
2850 |
CH2 symmetric stretching: lipids |
|
1650 |
Amide I peptide O=C–N–H stretching I α-helix |
|
1540 |
Amide–II
(N-H bend, C-N stretch)—predominately β-sheet |
|
1470 |
CH2 bending of the methylene chains in lipids |
|
1400 |
COO
-
symmetric stretching: fatty acids |
|
1310 |
CH2 stretching: Amide-III |
|
1230 |
PO2-
asymmetric stretching: nucleic acids and phospholipids |
|
1080 |
Symmetric
phosphate [PO2- (sym)] stretching:
collagen and phosphodiester nucleic acids. |
|
617 |
Ring
deformation of phenyl |
Table 2
|
Table 2 Dicofol Induced Changes in the FT-IR Band Areas Assigned to Different Biomolecules Present in Rat Testis |
||||
|
Wave number (cm−1) |
Experimental groups |
|||
|
Control |
5 mg/100gm BW |
7 mg/100gm BW |
10 mg/100gm BW |
|
|
3440 |
258.41 ± 22.09 |
217.71 ± 42.02 |
||
|
2920 |
3.62 ± 0.58 |
3.01 ± 0.62 |
2.88 ± 0.64** |
|
|
2850 |
1.03 ± 0.09 |
0.81± 0.03 |
||
|
1650 |
35.62 ± 0.33 |
24.54 ± 0.38 |
22.11 ± 0.86** |
|
|
1540 |
8.95 ± 0.98 |
7.72 ± 0.55 |
||
|
1470 |
0.31 ± 0.09 |
0.24 ± 0.08 |
||
|
1400 |
0.30 ± 0.08 |
0.23 ± 0.06 |
||
|
1310 |
0.22 ± 0.01 |
0.19 ± 0.06 |
||
|
1230 |
2.91 ± 0.06 |
2.56 ± 0.08 |
||
|
1080 |
3.10 ± 0.05 |
2.88 ± 0.04 |
||
4. CONCLUSION
In summary, our research showed that the histopathological components of the rat testis were significantly altered by exposure to higher doses of DCF (7 and 10 mg/100 gms of BW). Furthermore, it was evident from the results that the structure and composition of proteins had changed significantly. After a significant reduction in α helical structures and turns in the proteins, the intensity of the peaks ascribed to β-sheets increased in the rat testis intoxicated by high doses of DCF. There were observed aggregated β-sheet structures. Overall, the findings demonstrated that the testis was most susceptible to oxidative stress brought on by DCF intoxication.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
The authors are thankful to DST (Department of Science and Technology) New Delhi, for providing financial support through DST PURSE Phase II Program [F.No. SR/PURSE Phase 2/13(G)].
REFERENCES
Ahmad, A., & Ahmad, M. (2017). Deciphering the Toxic Effects of Organochlorine Pesticide, Dicofol on Human RBCs and lymphocytes. Pesticide Biochemistry and Physiology, 143, 127-134. https://doi.org/10.1016/j.pestbp.2017.08.007
Ahmad, M. F., Ahmad, F. A., Alsayegh, A. A., Zeyaullah, Md., AlShahrani, A. M., Muzammil, K., Saati, A. A., Wahab, S., Elbendary, E. Y., Kambal, N., Abdelrahman, M. H., & Hussain, S. (2024). Pesticides Impacts on Human Health and the Environment with their Mechanisms of Action and Possible countermeasures. Heliyon, 10(7), e29128. https://doi.org/10.1016/j.heliyon.2024.e29128
Ben Mukiibi, S., Nyanzi, S. A., Kwetegyeka, J., Olisah, C., Taiwo, A. M., Mubiru, E., Tebandeke, E., Matovu, H., Odongo, S., Abayi, J. J. M., Ngeno, E. C., Sillanpää, M., & Ssebugere, P. (2021). Organochlorine Pesticide Residues in Uganda's Honey as a Bioindicator of Environmental Contamination and Reproductive Health Implications to Consumers. Ecotoxicology and Environmental Safety, 214, 112094. https://doi.org/10.1016/j.ecoenv.2021.112094
Clark Jr., D. R., Spann, J. W., & Bunck, C. M. (1990). Dicofol (kelthane®)-induced Eggshell Thinning in Captive American Kestrels. Environmental Toxicology and Chemistry, 9(8), 1063-1069. https://doi.org/10.1002/etc.5620090813
David, M., Kartheek, R. M., & Manjunath, G. P. (2018). Acute and Sublethal Toxicity of Chlorpyrifos on Developmental Stages of Dattaphrynus Melanostictus. Journal of Applied Pharmaceutical Science, 8,(6), 087-093. https://doi.org/10.7324/JAPS.2018.8612
El-Kashoury, A. A., Salama, A. F., Selim, A. I., & Mohamed, R. A. (2010). Chronic Exposure Of Dicofol Promotes Reproductive Toxicity In Male Rats. 7(3).
Fujii, Y., Haraguchi, K., Harada, K. H., Hitomi, T., Inoue, K., Itoh, Y., Watanabe, T., Takenaka, K., Uehara, S., Yang, H.-R., Kim, M.-Y., Moon, C.-S., Kim, H.-S., Wang, P., Liu, A., Hung, N. N., & Koizumi, A. (2011). Detection of Dicofol and Related Pesticides in Human Breast Milk from China, Korea and Japan. Chemosphere, 82(1), 25-31. https://doi.org/10.1016/j.chemosphere.2010.10.036
Humason, G. L., & Humason, G. L. (1962). Animal Tissue Techniques (pp. 1-492). W.H. Freeman. https://doi.org/10.5962/bhl.title.5890
Jayaraj, R., Megha, P., & Sreedev, P. (2016). Organochlorine Pesticides, their Toxic Effects on Living Organisms and their Fate in the Environment. Interdisciplinary Toxicology, 9(3-4), 90-100. https://doi.org/10.1515/intox-2016-0012
Kartheek, R. M., & David, M. (2018). Assessment of Fipronil Toxicity on Wistar Rats: A Hepatotoxic Perspective. Toxicology Reports, 5, 448-456. https://doi.org/10.1016/j.toxrep.2018.02.019
Mello, M. L. S., & Vidal, B. C. (2012). Changes in the Infrared Microspectroscopic Characteristics of DNA Caused by Cationic Elements, Different Base Richness and Single-Stranded form. PloS One, 7(8), e43169. https://doi.org/10.1371/journal.pone.0043169
PubChem. (n.d.). Dicofol. Retrieved October 28, 2024
Qi, S.-Y., Xu, X.-L., Ma, W.-Z., Deng, S.-L., Lian, Z.-X., & Yu, K. (2022). Effects of Organochlorine Pesticide Residues in Maternal Body on Infants. Frontiers in Endocrinology, 13, 890307. https://doi.org/10.3389/fendo.2022.890307
Ramesh, H., & David, M. (2009). Respiratory Performance and Behavioral Responses of the Freshwater Fish, Cyprinus Carpio (Linnaeus) Under Sublethal Chlorpyrifos Exposure. Journal of Basic and Clinical Physiology and Pharmacology, 20(2), 127-139. https://doi.org/10.1515/jbcpp.2009.20.2.127
Shivanoor, S. M., & David, M. (2015). Fourier Transform Infrared (FT-IR) Study on Cyanide Induced Biochemical and Structural Changes in Rat Sperm. Toxicology Reports, 2, 1347-1356. https://doi.org/10.1016/j.toxrep.2015.10.004
Talari, A. C. S., Martinez, M. A. G., Movasaghi, Z., Rehman, S., & Rehman, I. U. (2017). Advances in Fourier transform infrared (FTIR) Spectroscopy of Biological Tissues. Applied Spectroscopy Reviews, 52(5), 456-506. https://doi.org/10.1080/05704928.2016.1230863
Tudi, M., Ruan, H. D., Wang, L., Lyu, J., Sadler, R., Connell, D., Chu, C., & Phung, D. T. (2021). Agriculture Development, Pesticide Application and Its Impact on the Environment. International Journal of Environmental Research and Public Health, 18(3), 1112. https://doi.org/10.3390/ijerph18031112
US EPA, O. (2013). 2006 IUR Resources [Other Policies and Guidance].
Vessela VitchevaDicofol (addendum). (2011). JMPR, 151-210-Google Search. (n.d.). Retrieved August 9, 2024
Zheng, Q., Li, J., Wang, Y., Lin, T., Xu, Y., Zhong, G., Bing, H., Luo, C., & Zhang, G. (2020). Levels and Enantiomeric Signatures of Organochlorine Pesticides in Chinese Forest soils: Implications for Sources and Environmental Behavior. Environmental Pollution, 262, 114139. https://doi.org/10.1016/j.envpol.2020.114139
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2024. All Rights Reserved.