
POSTERIOR REVERSIBLE ENCEPHALOPATHY SYNDROME (PRES) SECONDARY TO VASO-OCCLUSIVE CRISIS IN A SICKLE CELL ANEMIA PATIENT: A CASE REPORT
Osama Khider
Ahmed Elmansour 1![]() , Amna Ahmed 2
, Amna Ahmed 2![]() , Randa Abbas 3
, Randa Abbas 3![]() , Anas Mohamed 3
, Anas Mohamed 3![]() , Sabah Mohamed 3
, Sabah Mohamed 3![]() , Hibatalla Mohamed 3
, Hibatalla Mohamed 3![]() , Alwia Fadulalmola
3
, Alwia Fadulalmola
3![]() , Osman Ahmed 3, Mohammed
Naeem 4
, Osman Ahmed 3, Mohammed
Naeem 4![]() , Ahmed Hajhamed 3
, Ahmed Hajhamed 3![]() , Noura Abdelrazzig 3
, Noura Abdelrazzig 3![]() , Almothana Mohammedin
3
, Almothana Mohammedin
3![]() , Ahmed Babikir 5
, Ahmed Babikir 5![]()
1 Shendi
University, Department of Internal Medicine, Sudan
2 Shendi
University, Faculty of Medicine, Sudan
3 Ministry of Health, Sudan
4 Elsheikh Abdullah Elbadri University,
Faculty of Medicine, Sudan
5 Shendi University, Faculty of Medicine, Department of Clinical
Pathology, Sudan
|
|
ABSTRACT |
||
|
Background: Posterior Reversible Encephalopathy Syndrome (PRES) is a clinico-radiological diagnosis, characterized by distinctive neuroimaging features and non-specific neurological symptoms including: visual disturbances, altered consciousness, headache and seizures. The neuroimaging alterations are reversible and predominantly posterior in the parieto-occipital region. PRES is classically suspected in patients with severe hypertension, renal failure, autoimmune disorders, eclampsia, or immunosuppressant medications. Also patients with sickle cell disease are exposed to different forms of brain insults as part of their disease process. So far, PRES has been reported in a few patients with sickle cell disease with some of these patients having recurrent episodes. Case: This case report presents a 35-years-old Sudanese male known case of Sickle Cell Disease presenting with a full presenting picture of PRES included sudden onset of headache, seizures, visual disturbances, and altered consciousness. While the patient had exhibited near-all clinical traits of SCD, it was the vaso-occlusive crisis -in form of priapism- that the most evident of symptoms and signs which had evolved to PRES. Discussion: Few hypotheses have been established regarding the pathophysiology of PRES. One believes that the sudden onset of hypertension causes breakdown in brain autoregulation especially in the occipital area. This leads to hyperperfusion and subsequent extravasation of proteins and fluids, forming a local vasogenic oedema. Another suggests that the endothelial dysfunction due to sepsis and eclampsia is the culprit mechanism of injury despite its association with ischemia and vasospasm. Patients with sickle cell disease are exposed to different forms of brain insults as part of their disease process, PRES has been reported in a few patients with sickle cell disease with some of these patients having recurrent episodes. We are reporting this unique case sickle cell disease and PRES to further highlight the coexistence of the two conditions. Conclusion: This study highlights the potential association between sickle
cell disease and the development of PRES. As it is well explained as a result
from the vaso-occlusive crisis that takes place in
brain blood vessels which leads to hypo-perfusion of the brain and thus brain
ischemia, also explained by the endothelial injury in PRES-associated
conditions which may lead to vascular instability and vasoconstriction. |
|||
|
Received 12 February
2024 Accepted 13 March 2024 Published 31 March 2024 Corresponding Author Ahmed Babikir, ahmedbabikir@ush.edu.sd DOI 10.29121/granthaalayah.v12.i3.2024.5419 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2024 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Posterior Reversible Encephalopathy
Syndrome, Sickle Cell Anemia, PRES, Brain Edema |
|||
1. INTRODUCTION
Posterior Reversible Encephalopathy Syndrome (PRES) is a clinic-radiological diagnosis with distinctive neuroimaging features and non-specific neurological symptoms including: visual disturbances, altered consciousness, headache and seizures. The neuroimaging alterations are reversible and predominantly posterior Hinchey et al. (1996). PRES was firstly described by Hinchy et al in 1996 when it was identified as reversible posterior leukoencephalopathy syndrome Hinchey et al. (1996). Several terms were suggested there after to describe the condition till finally in 2000 the term Posterior Reversible Encephalopathy Syndrome was proposed by Casey et al, who defined it as acute and subacute onset of headache and manifestations of focal neurological signs Casey et al. (2000). Posterior Reversible Encephalopathy Syndrome (PRES) was found in the context of certain conditions mainly hypertension, eclampsia, renal failure, some autoimmune diseases and the use of immunosuppressant and chemotherapy agents Triplett et al. (2022). PRES can be encountered at any age from infants to elderly, but most commonly occur in young or middle-aged adults with a mean age of 45 years Fugate et al. (2010), Lee et al. (2008). Among adult population, PRES has been reported in 98% of patients with eclampsia Brewer et al. (2013), in 2.7%-25% of patients following bone marrow transplantation Reece et al. (1991), Bartynski et al. (2004), in 0.4%-6% of patients following solid organ transplantation Bartynski et al. (2008), 0.84% of patients with end stage renal disease Canney et al. (2015), and 0.69% of patients with systemic lupus erythromatosis Lai et al. (2013). Patients with sickle cell disease are exposed to different forms of brain insults as part of their disease process, with up to 11% of young patients below 20 years of age showed definite stroke, while up to 30% would display evidence of silent cerebral infarctions Ohene-Frempong (1998), Switzer et al. (2006), Miller et al. (2001). So far, PRES has been reported in a few patients with sickle cell disease with some of these patients having recurrent episodes Nair & Testai (2011). We are reporting this unique case sickle cell disease and PRES to further highlight the coexistence of the two conditions.
2. Case Presentation
A 35-years-old Sudanese male known case of Sickle Cell anemia with history of heavy, serial blood transfusion was presented to the emergency room with severe headache, periorbital and facial edema, blurring of vision, confusion, and projectile vomiting, and generalized tonic- colonic seizures.
His past medical history revealed that 11 days prior to his current admission, the patient had suffered from sudden onset of headache, located mainly in the occipital area, dull and constant, with radiation to neck and shoulders. Aggravated by coughing, sneezing, and straining, not relieved by analgesia, accompanied by frequent attacks of visual deficits ranging from aumarosis fugax to complete loss of vision lasting less than 15 minutes.
The headache had subside using imitriptrine tablets, 4 days before the recent admission the patient had presented with a Sickle Cell vaso-occlusive crisis in a form of priapism, one day prior the admission, the patient presented to the emergency department with generalized tonic- colonic seizure, which treated by diazepam 10mg and phenytoin 750 mg loading, and 4L fluid infusion, on that same day the patient started to vomit large amount of projectile vomiting 3 times per day, not proceeded by nausea and not related to food intake. As well as he had suffered from blurring of vision and attacks of hemianopia lasted for less than two minutes. On the day of admission, The examination upon admission revealed confusion with GCS 9/15, disorientation and blood pressure of 200/100 mm Hg. The patient suffered from 4 attacks of generalized tonic colonic seizures, each attack lasted for less than 2 minutes accompanied by rolling up of the eyes and drooling of saliva, in the 3rd attack the patient had passed urine. After each attack the patient was drowsy, he had been treated by diazepam 10mg and phenytoin 750 mg loading and 100mg of phenytoin in 100 ml of Normal Saline. The patient also developed periorbital and facial edema. the patient continued to vomit large amount of projectile vomiting 3 times per day, not proceeded by nausea and not related to food intake and on the 4th day of admission patient had developed very severe attack of headache with blurring of vision which had progressed and deteriorated to complete loss of vision, which last for only 1 hour, funduscopic examination revealed bilateral disc oedema.
Peripheral blood picture identified extensive sickling pattern with reduction of RBC mass; Figure 1. Further, laboratory tests revealed normal renal function and serum electrolytes, liver function. his hemoglobin and platelet count were 4.7 g/dL and 408,000/mm3, respectively, white blood cell count was elevated at 21,500/mm3; blood, urine cultures, and serology tests (HIV, hepatitis C, B) produced negative results.
Figure 1
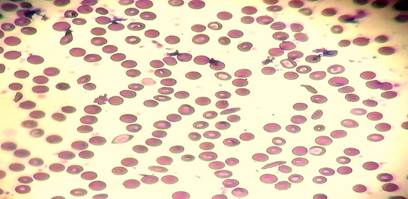
|
Figure 1 Showing Peripheral Blood Picture Showing Extensive Sickling Pattern, Variant Degrees of Spherocytosis, Iron Deficiency Anemia and Reticulocytosis. |
Brain imaging via computed tomography showed bilateral, symmetrical, diffuse hemispheric edema; primarily involving the parietal and occipital regions of the brain; Figure 2. The diagnosis of Posterior Reversible Encephalopathy Syndrome secondary to hypertension, sickling crisis and fluid overload was made, and treatment was initiated with the above-outlined therapy.
Figure 2
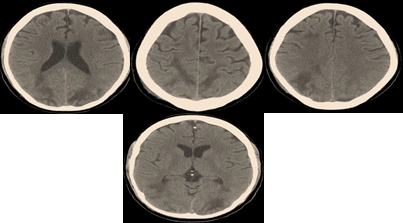
|
Figure 2 Showing CT-Brain Imaging with Extensive, Bilateral, Symmetrical Parieto-Occipital Hypo-Attenuating Lesions; Resembling Diffuse Vascular Edema of the Brain Tissue |
3. Discussion
We are reporting A 35-years-old Sudanese male known case of Sickle Cell Anemia presenting with a full presenting picture of Posterior Reversible Encephalopathy Syndrome (PRES), while our patient had exhibited near-all clinical traits of SCA, it was the vaso-occlusive crisis –in form of Priapism- that the most evident of symptoms and signs which had evolved to PRES.
Since the endothelial damage is playing a major part in the cerebrovascular disease observed in Sickle Cell Anemia, and as PRES happens mainly due to endothelial dysfunction which leads to cerebro-vascular autoregulation dysfunction and vasogenic edema Frye (2009), it has been reported that patients with SCA are at high risk of developing posterior reversible encephalopathy syndrome (PRES) Frye (2009).
Priapism is a serious complication of sickle cell disease, which happens due to vaso-occlusive crisis that affects the small and medium vessels of the penis, it is reported in nearly more than the half of males with sickle cell disease Tarry et al. (1987), Edmond et al. (1980), our patient had suffered from multiple episodes of priapism had been managed variously starting from hydration and analgesia up to simple and partial exchange transfusion to relieve the pain and preserve sexual functions Seeler (1973), Walker et al. (1983). Priapism and acute neurologic events are complications of sickle cell disease that have been considered to be rarely happened and unrelated, yet there are some cases which reported to have an acute, severe, transient neurological problems after Priapism partial exchange transfusion therapy Rackoff (1992). this can be explained as the arterial circulation of the brain is very common site of intimal hyperplasia and partial vascular occlusion in sickle cell disease. Thus, these vessels are perfect sites of attachment for adhesive plasma proteins, activated platelets, and residual sickle cells Francis & Johnson (1991), Hebbel (1991), which leads to an increase in the levels of adhesive plasma proteins, especially Von Willebrand factor and fibrinogen and also decrease levels of Protein S Francis & Johnson (1991), Francis (1988).
As our patient also suffered from sudden onset of headache, in the occipital area, dull and constant, with radiation to neck and shoulders, it is known that headache is actually a very Headache is a very common neurological complications found in SCA patients Niebanck et al. (2007), this happens mainly due to the presence of poor cerebral vessel auto-regulation seen in SCA patients, which leads to cerebral vasodilation without any increase in the blood flow Nath et al. (2000), and that vasodilatation is the cause of the headache.
While clinical presentations of PRES are characterized by acute or subacute onset of neurological symptoms Hinchey et al. (1996), it was headache the most common symptom reported Li et al. (2020). according to a recent study Headache develops in up to half of patients with PRES Legriel et al. (2012), and it is almost always dull, diffuse, and gradual in onset as well described and presented in our patient.
Seizures are the most common and frequent clinical presentation seen in PRES Approximately up to 81% Legriel et al. (2012), Lee et al. (2008), Garg (2001). in the majority of cases seizures were generalized tonic-clonic seizures Spencer (2015) as perfectly well seen in our patient, yet it can range from absence seizures to convulsive seizures. It might be seen as focal onset with following secondary generalization or generalized from the very first moment. Multiple seizures and status epilepticus (SE) are also frequent Brewer et al. (2013), Servillo et al. (2007). and typically occur within the first and second days of presentation Datar et al. (2015), Kastrup et al. (2012).
Visual disturbance in PRES is very common and they vary from blurred vision, hemianopia to complete cortical blindness Brewer et al. (2013), Servillo et al. (2007). Those visual problems are mainly due to endothelial damage of the optic nerve and the vasogenic edema of the brain Frye (2009). As our patient had developed very severe attack of headache combined by blurring of vision which had progressed and deteriorated to complete loss of vision, which last for only 1 hour, funduscopy revealed he had disc edema.
Despite that PRES often affects the occipital regions, only 39% of patients whom suffered from PRES reported visual symptoms Legriel et al. (2012), Brewer et al. (2013). Those complained from reduced visual acuity, diplopia, visual field problems, cortical and temporal blindness, color vision abnormality and visual hallucinations, also in very recent study patients with PRES visual complaints included; complaints bi-lateral vision loss in the majority of patients (64%), diplopia in (27%), and unilateral vision loss, color abnormality, and pain with extraocular movements each individually found in (9%) of patients Lifson et al. (2019).
Examination upon admission of the patient revealed confusion with GCS 9/15 and disorientation, confusion is well explained in our patient as a result from the vaso-occlusive crisis that takes place in brain blood vessels which leads to hypoperfusion of the brain and thus brain ischemia, also explained by the endothelial injury in PRES-associated conditions which may lead to vascular instability and vasoconstriction, with subsequent hypoperfusion, Bartynski (2008), Faraci & Heistad (1998) and hence decrease the level of consciousness present in PRES and Sickle cell brain vaso-occlusive crisis is seen.
4. Conclusion
We a report a peculiar case of Posterior Reversible Encephalopathy Syndrome (PRES) in a sickle cell patient to highlight the potential association between sickle cell vaso-occlusive crisis in the neuronal vascular beds which leads to hypo-perfusion of the brain and thus brain ischemia, and associated endothelial injury with subsequent development of the unique phenomenon of PRES.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
Bartynski, W. S., Tan, H. P., Boardman, J. F., Shapiro, R., & Marsh, J. W. (2008). Posterior Reversible Encephalopathy Syndrome After Solid Organ Transplantation. AJNR. American Journal of Neuroradiology, 29(5), 924-930. https://doi.org/10.3174/ajnr.A0960
Bartynski, W. S., Zeigler, Z. R., Shadduck, R. K., & Lister, J. (2004). Pretransplantation Conditioning Influence on the Occurrence of Cyclosporine or FK-506 Neurotoxicity in Allogeneic Bone Marrow Transplantation. AJNR. American Journal of Neuroradiology, 25(2), 261-269.
Bartynski, W.S. (2008). Posterior Reversible Encephalopathy Syndrome, Part 2: Controversies Surrounding Pathophysiology of Vasogenic Edema. AJNR Am J Neuroradiol, 29(6), 1043-1049. https://doi.org/10.3174/ajnr.A0929
Brewer, J., Owens, M. Y., Wallace, K., Reeves, A. A., Morris, R., Khan, M., LaMarca, B., & Martin, J. N. (2013). Posterior Reversible Encephalopathy Syndrome in 46 of 47 Patients with Eclampsia. American Journal of Obstetrics and Gynecology, 208(6). e1-468.e4686. https://doi.org/10.1016/j.ajog.2013.02.015
Canney, M., Kelly, D., & Clarkson, M. (2015). Posterior Reversible Encephalopathy Syndrome in End- Stage Kidney Disease: Not Strictly Posterior or Reversible. American Journal of Nephrology, 41(3), 177-182. https://doi.org/10.1159/000381316
Casey, S.O., Sampaio, R.C., Michel, E., & Truwit, C.L. (2000). Posterior Reversible Encephalopathy Syndrome: Utility of Fluid-Attenuated Inversion Recovery MR Imaging in the Detection of Cortical and Subcortical Lesions. AJNR Am J Neuroradiol, 21(7), 1199-206.
Datar, S., Singh, T., Rabinstein, A.A. (2015). Long- Term Risk of Seizures and Epilepsy in Patients with Posterior Reversible Encephalopathy Syndrome. Epilepsia, 56, 564-8. https://doi.org/10.1111/epi.12933
Edmond, A.M., Holman, R., Hayes, R. J., & Serjeant, G.R. (1980). Priapism and Impotence in Homozygous Sickle Cell Disease. Arch Intern Med, 140, 1434-7. https://doi.org/10.1001/archinte.1980.00330220022011
Faraci, F.M., & Heistad, D.D. (1998). Regulation of the Cerebral Circulation: Role of Endothelium and Potassium Channels. Physiol Rev, 78(1), 53-97. https://doi.org/10.1152/physrev.1998.78.1.53
Francis, R.B. (1988). Protein S Deficiency in Sickle Cell Anemia. J Lab Clin Med, 111, 571-6.
Francis, R.B., & Johnson, C.S. (1991). Vascular Occlusion in Sickle Cell Disease: Current Concepts and Unanswered Questions. Blood, 77, 1405-14. https://doi.org/10.1182/blood.V77.7.1405.1405
Frye, R.E. (2009). Reversible Posterior Leukoencephalopathy Syndrome in Sickle-Cell Anemia. Pediatr Neurol, 40(4), 298-301. https://doi.org/10.1016/j.pediatrneurol.2008.10.024
Fugate, J. E., Claassen, D. O., Cloft, H. J., Kallmes, D. F., Kozak, O. S., & Rabinstein, A. A. (2010). Posterior Reversible Encephalopathy Syndrome: Associated Clinical and Radiologic Findings. Mayo Clinic Proceedings, 85(5), 427-432. https://doi.org/10.4065/mcp.2009.0590
Garg, R.K. (2001). Posterior Leukoencephalopathy Syndrome. Postgrad Med J, 77(903), 24-28. https://doi.org/10.1136/pmj.77.903.24
Hebbel, R. P. (1991). Beyond Hemoglobin Polymerization: the Red Blood Cell Membrane and upathophysiology. Blood, 77, 214-37. https://doi.org/10.1182/blood.V77.2.214.214
Hinchey, J., Chaves, C., Appignani, B., Breen, J., Pao, L., Wang, A., Pessin, M. S., Lamy, C., Mas, J. L., & Caplan, L. R. (1996). A Reversible Posterior Leukoencephalopathy Syndrome. The New England journal of medicine, 334(8), 494-500. https://doi.org/10.1056/NEJM199602223340803
Kastrup, O., Gerwig, M., & Frings, M. (2012). Posterior Reversible Encephalopathy Syndrome (PRES): Electroencephalographic Findings and Seizure Patterns. J Neurol, 259, 1383-9. https://doi.org/10.1007/s00415-011-6362-9
Lai, C. C., Chen, W. S., Chang, Y. S., Wang, S. H., Huang, C. J., Guo, W. Y., & Huang, D. F. (2013). Clinical Features and Outcomes of Posterior Reversible Encephalopathy Syndrome in Patients with Systemic Lupus Erythematosus. Arthritis Care & Research, 65(11), 1766-1774. https://doi.org/10.1002/acr.22047
Lee, V. H., Wijdicks, E. F., Manno, E. M., & Rabinstein, A. A. (2008). Clinical Spectrum of Reversible Posterior Leukoencephalopathy Syndrome. Archives of Neurology, 65(2), 205-210. https://doi.org/10.1001/archneurol.2007.46
Legriel, S., Schraub, O., Azoulay, E., Hantson, P., Magalhaes, E., Coquet, I. (2012). Determinants of Recovery from Severe Posterior Reversible Encephalopathy Syndrome. PLoS One, 7(9), e44534. https://doi.org/10.1371/journal.pone.0044534
Li, K., Yang, Y., Guo, D., Sun, D., & Li, C. (2020). Clinical and MRI Features of Posterior Reversible Encephalopathy Syndrome with Atypical Regions: A Descriptive Study with a Large Sample Size. Front Neurol, 11, 194. https://doi.org/10.3389/fneur.2020.00194
Lifson, N., Pasquale, A., Salloum, G., & Alpert, S. (2019). Ophthalmic Manifestations of Posterior Reversible Encephalopathy Syndrome. Neuro-Ophthalmology, 43(3), 180-4. https://doi.org/10.1080/01658107.2018.1506938
Miller, S. T., Macklin, E. A., Pegelow, C. H., Kinney, T. R., Sleeper, L. A., Bello, J. A., DeWitt, L. D., Gallagher, D. M., Guarini, L., Moser, F. G., Ohene-Frempong, K., Sanchez, N., Vichinsky, E. P., Wang, W. C., Wethers, D. L., Younkin, D. P., Zimmerman, R. A., DeBaun, M. R., & Cooperative Study of Sickle Cell Disease (2001). Silent Infarction as a Risk Factor for Overt Stroke in Children with Sickle Cell Anemia: A Report from the Cooperative Study of Sickle Cell Disease. The Journal of Pediatrics, 139(3), 385-390. https://doi.org/10.1067/mpd.2001.117580
Nair, A., & Testai, F. D. (2011). Recurrent Posterior Reversible Encephalopathy Syndrome in a Sickle Cell Patient. Journal of the National Medical Association, 103(2), 170-172. https://doi.org/10.1016/s0027-9684(15)30267-4
Nath, K.A., Shah, V., Haggard, J.J. (2000). Mechanisms of Vascular Instability in a Transgenic Mouse Model of Sickle Cell Disease. Am J Physiol Regul Integr Comp Physiol, 279, R1949-R1955. https://doi.org/10.1152/ajpregu.2000.279.6.R1949
Niebanck, A. E., Pollock, A. N., Smith-Whitley, K., Raffini, L. J., Zimmerman, R. A., Ohene-Frempong, K., & Kwiatkowski, J. L. (2007). Headache in Children with Sickle Cell Disease.-Prevalence and Associated Factors. J Pediatr, 1(1), 67-72. https://doi.org/10.1016/j.jpeds.2007.02.015
Ohene-Frempong, K., Weiner, S. J., Sleeper, L. A., Miller, S. T., Embury, S., Moohr, J. W., Wethers, D. L., Pegelow, C. H., & Gill, F. M. (1998). Cerebrovascular Accidents in Sickle Cell Disease: Rates and Risk Factors. Blood, 91(1), 288-294.
Rackoff, W. (1992). Neurologic Events After Partial Exchange Transfusion for Priapism in Sickle Cell Disease. The Journal of Pediatrics, 120(6), 882-885. https://doi.org/10.1016/S0022-3476(05)81954-7
Reece, D. E., Frei-Lahr, D. A., Shepherd, J. D., Dorovini-Zis, K., Gascoyne, R. D., Graeb, D. A., Spinelli, J. J., Barnett, M. J., Klingemann, H. G., & Herzig, G. P. (1991). Neurologic Complications in Allogeneic Bone Marrow Transplant Patients Receiving Cyclosporin. Bone Marrow Transplantation, 8(5), 393-401.
Seeler, R.A. (1973). Intensive Transfusion Therapy for Priapism in Boys with Sickle Cell Anemia. J Urol, 110, 360-1. https://doi.org/10.1016/S0022-5347(17)60217-9
Servillo, G., Bifulco, F., & De Robertis, E. (2007). Posterior reversible encephalopathy syndrome in intensive care medicine. Intensive Care Med, 33(2), 230-236. https://doi.org/10.1007/s00134-006-0459-0
Spencer, D. (2015). PRES-ing for aNswers About Long-Term Seizure Risk in Patients with Posterior Reversible Encephalopathy Syndrome: PRES-ing for Answers About Long-Term Seizure Risk. Epilepsy Currents, 15(6), 317-8. https://doi.org/10.5698/1535-7511-15.6.317
Switzer, J. A., Hess, D. C., Nichols, F. T., & Adams, R. J. (2006). Pathophysiology and Treatment of Stroke in Sickle-Cell Disease: Present and Future. The Lancet. Neurology, 5(6), 501-512. https://doi.org/10.1016/S1474-4422(06)70469-0
Tarry, W.F., Duckett, J.W., & Snyder, H.M. (1987). Urological Complica- Tions of Sickle Cell Disease in a Pediatric Population. J Urol, 138, 592-4. https://doi.org/10.1016/S0022-5347(17)43267-8
Triplett, J.D., Kutlubaev, M.A., Kermode, A.G., & Hardy, T. (2022). Posterior Reversible Encephalopathy Syndrome (PRES): Diagnosis and Management Practical Neurology, 22, 183-189. https://doi.org/10.1136/practneurol-2021-003194
Walker, E.M., Mitchum, E.N., Rous, S.N., Glassman, A.B., Cannon, A., & McInnes, B.K. (1983). Automated Erythrocytopheresis for Relief of Priapism in Sickle Cell Hemoglobinopathies. J Urol, 130, 912-6. https://doi.org/10.1016/S0022-5347(17)51573-6
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2024. All Rights Reserved.