
Proposed Mechanism for Breast Cancer Metastasis: The Piercing and Suctioning of Cancer Cells Material into the Intercellular Space During Crystallization
1 Citizen
Scientist, 13442 SW 102nd Lane Miami, Florida USA
|
|
ABSTRACT |
||
|
The purpose of
this manuscript is to introduce a hypothesis correlating the process of
hydroxyapatite crystallization as a factor allowing for the transfer of
intracellular breast cancer matter into the intercellular space by rupturing
cellular outer membranes. In addition, this matter could also be transferred
into the lymphatic system to be disseminated, thus metastasis. The hypothesis
is supported by published in vitro experiments where during crystallization
lipid cells are being ruptured by advancing crystals. As fluid evaporates
during crystallization, a Backwards Suction (BS) phenomenon of cells and
debris has been also documented to occur during crystals formation of the
anisotropic Potassium Ferricyanide when within approximately 1 mm of human
tissue. This BS during crystals formation is herein hypothesized to be a
mechanism dislodging tissue in type II ductal fragile malignant breast cancer
tissue. The cellular material would then be pierced by the crystals and
suctioned by the lymphatic circulation with its consequences. |
|||
|
Received 30 June 2023 Accepted 31 July 2023 Published 15 August 2023 Corresponding Author Anjay
Kumar Mishra, anjaymishra2000@gmail.com DOI 10.29121/granthaalayah.v11.i7.2023.5237 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2023 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Crystallization Factor, Backwards
Suction, Microcalcifications Genesis, Breast Cancer Metastasis,
Hydroxyapatite, Lymph Nodes GLOSSARY Anisotropy: Anisotropy is the property of being directionally
dependent, as opposed to isotropy, which means homogeneity in all directions. Backwards Suction: Matter suctioned at end of crystallization. K3Fe: Acronym for
Potassium Ferricyanide. Paramagnetic: Attraction to incoming electromagnetic radiation ie: Potassium Ferricyanide. SSP: Single Slide
Preparation. Matter to be tested placed on surface of glass slide. |
|||
1. INTRODUCTION
The presence of calcified matter observed in breast
biopsies, are classified by their physical size, and having unknown origins, to
the point of researchers attributing the calcification material itself as a
factor in enhancing malignant potentials Haka et al. (2002). In this
manuscript basic science in vitro experiments are presented proposing a
mechanism supporting microcalcifications in ductal breast cancer type II tissue
as essential for metastasis, as follows:
2. In Vitro Backwards Suction During Crystals Formation
The images and video shown below are representative
of the Backwards Suction Phenomenon. This was reported and hypothesized at the
time to be a factor in the genesis of coronary artery disease (Figure 2, Figure 3), the additional figures (Figure 4, Figure 5, Figure 6, Figure 7, Figure 8) demonstrate the
backwards suction effect on tissue, supporting the effect of crystals piercing
lipids membranes, thereby the proposed hypothesis Embí (2020).
2.1. The Hypothesis Stated
“Hydroxyapatite crystals
are a malignancy enhancement agent in type II ductal breast carcinomas, by
piercing cells’ outer membranes and spilling material into the intercellular
space. This material could then be transported by the lymphatic system with its
consequences.” (Diagram Figure 1)
2.2. PROPOSED DUCTAL BREAST TYPE II CARCINOMA METASTASIS UNDERLYING MECHANISM
Figure 1
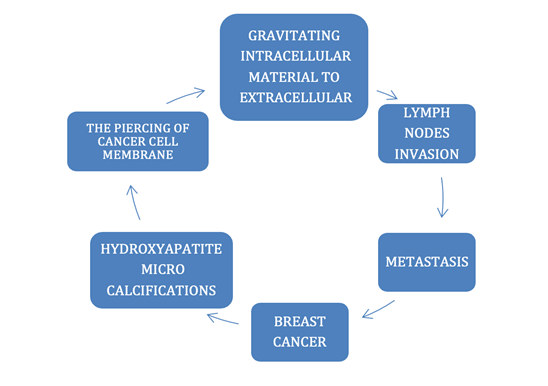
|
Figure 1 Proposed Mechanism for Breast Cancer Ductal Type II Metastasis. |
3. MATERIALS AND METHODS
3.1. MATERIALS
1) Potassium Ferricyanide Crystal. K3Fe
(CN)6.
CSA # 13746-66-2.
2) Hair Follicles plucked via tweezers from author’s scalp
3) Microscope glass slides: 25x75x1mm thickness. Pearl Cat. No.
7101
4) Room relative humidity monitored by an ACU-RITE sensor model
# 01536
5) Digital Video Microscope Celestron II
model # 44341, California, USA.
6) Images downloaded to an Apple Computer MacBook Pro Photo
Application.
7) Human lipid droplets.
8) Lizard tail lipid droplets.
3.2. METHODS
3.2.1. PREPARING THE SOLUTION
A solution was prepared by
diluting ≅ 2 grams of Potassium Ferricyanide (K3Fe) crystals
in 2 ml of the previously tested for impurities bottled spring water. The
solution was placed inside a 6-inch 4 mm OD glass tube and withdrawn as needed.
3.2.2. THE SINGLE SIDE PREPARATION (SSP)
The SSP is an open-air technique where
freshly plucked in toto human hairs were placed on a clean
25x75x1mm glass slide; and covered by drops of K3Fe in
solution; the liquid was then allowed to evaporate. Prior to evaporation, the
drops were gently touched by a wooden toothpick and dispersed to cover the
follicle and shaft. After the hair sample stops drifting and stabilizes, a
clean wooden toothpick was used to gently shepherd the hair sample away from
the drop edges. As evaporation starts, images and video recordings are recorded
and stored.
4. PROCEDURES
Spontaneous detachment of a small
lizard tail allowed for placing small segments on a glass slide. Two drops of
diluted Potassium Ferricyanide in water covered the sample, as the Ferricyanide
evaporated, crystals formed, and some penetrated the lipid samples (Figure 5, Figure 6, Figure 7, Figure 8, Figure 9). The
figures showing human lipid droplets were reproduced from previous papers (Figure 2, Figure 3, Figure 4).
4.1. Demonstration of externally trapped attracted solid human tissue particles during crystals formation.
Since most breast
cancers are classified as solid tumors; and human
hair follicles are a cohesive solid miniorgan, the
process of crystals formation near solid tissue has been demonstrated to
suction cellular material from hair follicles. The tissue particles are
documented being trapped by the crystals (Figure 2, Figure 3), creating a complex type of crystals.
The hypothesized “complex type” of hydroxyapatite (HA) reported in Type II
breast cancer is supported by published evidence as stated: “Although type II
microcalcifications are primarily composed of calcium hydroxyapatite, they also
contain trace amounts of several biological impurities…… On the basis of
these results, we believe that type II microcalcifications formed in benign
ducts typically contain a larger amount of calcium carbonate and a smaller
amount of protein than those formed in malignant ducts” (Cox and Morgan
(2013), Gosling et al. (2019)). Based on the data herein presented, it
could be stated that the type II calcifications contain externally trapped
material (between crystals) (Figure 4).
4.2. PRIOR ACTUAL
PUBLISHED IMAGES AND VIDEO RECORDINGS
Figures 2,3,4 reproduced from: Abrahám A. Embí BS MBA. (2020). Introducing Crystallization Backward Suction
Trapping Lipids and Debris as Proposed Additional Factor in The Genesis of
Coronary Artery Disease. International Journal of Research -GRANTHAALAYAH,
8(9), 215-233. https://doi.org/10.29121/granthaalayah.v8.i9.2020.1174
Figure 2
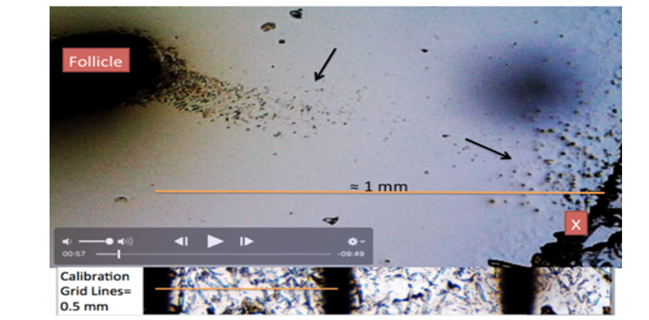
|
Figure 2 Showing Lower Right Corner X: Video-Frame 00:57”. Crystals Formation Attracting Particles from Human Tissue (Hair Follicle). |
Figure 3

|
Figure 3 Amplified Image Showing the Process of Crystallization When Near Solid Human Tissue Attracting Particles (Lipids and Debris). Frame 1:15 of Video Recording Showing Hair Follicle Molecules Attracted Towards Evaporating Potassium Ferricyanide During Crystals Growth Stage. For Additional Video Details Please Link to: https://youtu.be/Kv1rRdNwuF4 |
Figure 4
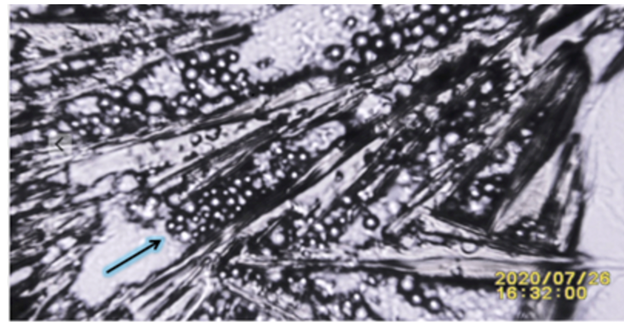
|
Figure 4 Image from SSP in Vitro Experiment Where During the
Process of Crystallization of Potassium Ferricyanide When Close to Solid
Human Tissue (Hair Follicle) Particles are Suctioned and Trapped.
Hypothesized to also Occur from Malignant Ducts Crystals. Supporting
Observation Addressed in References (Haka et al. (2002), Cox and Morgan (2013)) as follows: “On the basis of these results, we believe that
type II microcalcifications formed in benign ducts typically contain a larger
amount of calcium carbonate and a smaller amount of protein than those formed
in malignant ducts.” |
4.3. Figure 5, Figure 6, Figure 7 FROM
PREVIOUS RESEARCH DEMONSTRATING CRYSTAL PIERCING LIPID DROPLET MEMBRANE
Embi AA.
(2023) Introducing Electromagnetic Energy from
Hydrocolloid Wound Dressing Paste Penetrating a Glass Barrier Disrupting Human
Skin Lipid Droplets Size and Membranes: Possible Implications in Cancer Cells
Genesis and/or Cure. International Journal Research Granthaalayah. 11(2),
47-54. doi: 10.29121/ Granthaalayah.v11. i2.2023.5032
Figure 5
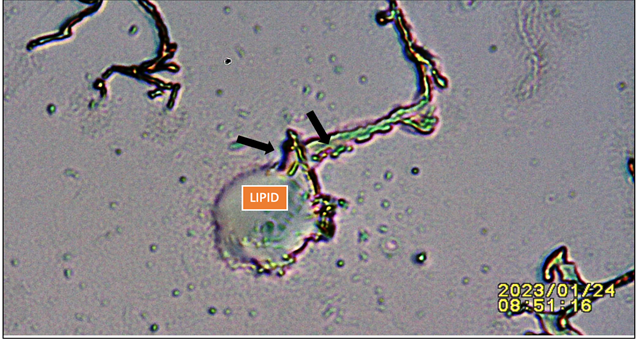
|
Figure 5 Showing Lipid Droplet Punctured by Advancing Potassium Ferricyanide Crystal. Black Arrows: Pointing at Lipid Droplet Draining into Advancing Crystal. |
Figure 6
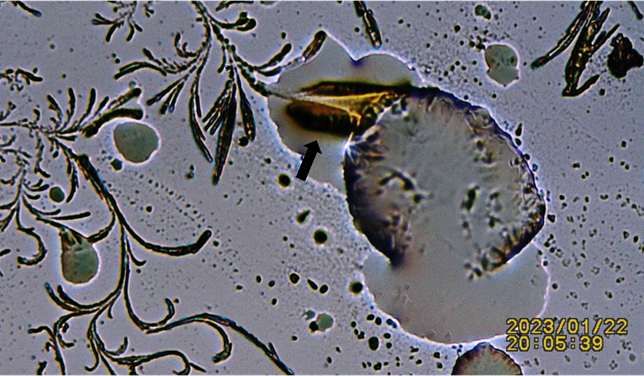
|
Figure 6 Additional Figure Showing Advancing Crystals Perforating Lipid Droplet. Black Arrow: Notice the Appearance of an Electrical Discharge between Lipid Droplet and Advancing Crystal. |
Figure 7
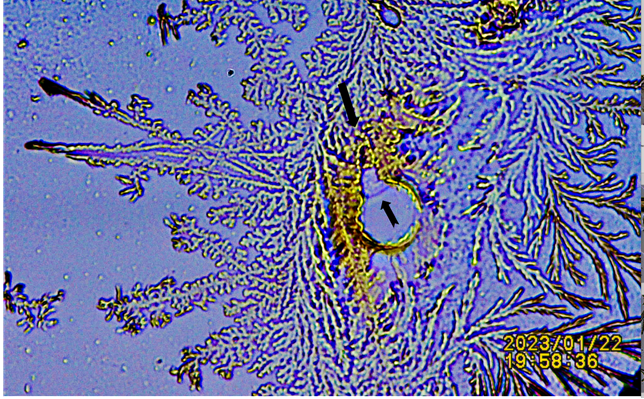
|
Figure 7 Additional Demonstration of Lipid Droplet Trapped and Perforated by Crystals. Notice the Staining of Crystals Possibly Caused by Spilled Lipid Fluid. |
4.4. The Following
Images (Figure 8, Figure 9) Also
Reproduced from Previous Research.
Embi, A. A. (2022). Introducing Methodology to Detect
Dead Tissue Stored Energy. International Journal of Research -
GRANTHAALAYAH, 10(8), 20–29. doi: 10.29121/granthaalayah.v10.i8.2022.4733
Figure 8
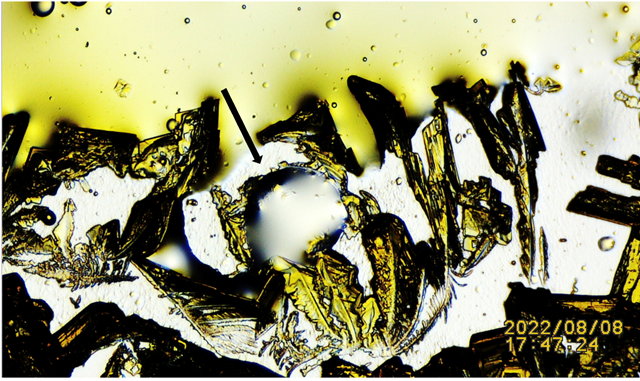
|
Figure 8 N:1 Black Arrow: Pointing at Potassium Ferricyanide Crystals Penetrating and Spilling Harvested Lizard’s Lipid Droplet. For Additional Details Link to: Video link https://youtu.be/zoPhBH_-fHc |
Figure 9
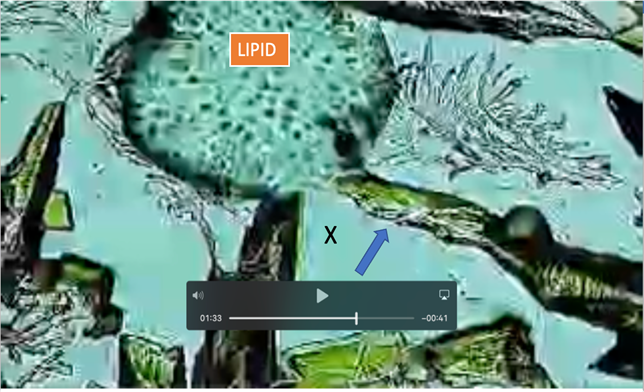
|
Figure 9 N;2 Frame 01:33 from Video Showing: Blue Arrow: Amplified Potassium Ferricyanide Crystals Attracted to Lipid Droplet Perforating Membrane. X: Spilled Intralipid Material. for Additional Details link to: https://youtu.be/zoPhBH_-fHc |
Exhibit 1
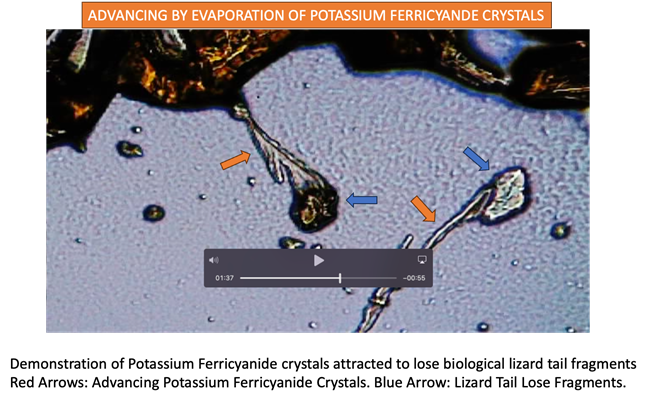
|
Exhibit 1 Additional Experiment Demonstrating Affinity Of Potassium Ferricyanide Crystals Towards Lose Tissue Fragments. Please
link to video frames 1:21 thru 1.37 for further details. Please
note when crystals attached to tissue triggering a noticeable spontaneous
energy discharge to the point of changing image depth of field
(focusing. |
5. SUMMARY
The data presented in this document supports a hypothesis
whereby the process of Hydroxyapatite crystallization in breast tissue induces
a backwards suction attracting detached breast cancer cells that are then
pierced causing it to shed protein and biological impurities into the
intercellular space. This crystallization process was duplicated in vitro
using liquid Potassium Ferricyanide and selecting freshly plucked human tissue
(hair follicles) as sentinels. Potassium Ferricyanide was chosen due to the
crystal’s pointed tip mimicking hydroxyapatite crystal, thus able to penetrate
cells membranes, as well as having the property of absorbing incoming energy
(electromagnetic) as shown in (Figure 8, Figure 9), where crystals
are attracted to the energy from lipid droplets. In addition, hydroxyapatite,
and Potassium Ferricyanide are both classified as being paramagnetic and
anisotropic, thus adding credence to the stated hypothesis in this manuscript (B. Viswanath et al. (2007), B. N. Figgis et al. (1969)). Those
experiments also support published evidence where in breast cancer tissue the
calcification “crystallite size and non-uniform strain normal to basal planes
increased significantly with malignancy”. Additionally, the findings herein
presented could also support a published notion of Hydroxyapatite crystals as a
malignancy enhancement agent He et al. (2019). Occurring by piercing breast cancer
cells and spilling its contents in the intercellular space as shown in Figure 5, Figure 6, Figure 7, Figure 8, Figure 9.
Please also note Exhibit 1 demonstrating advancing Potasium Ferricyanide crystals being selectively attracted
towards biological tissue droplets. This material could then be transported by the lymphatic
system with its consequences.
Additionally, a second statement could be stated as follows: “Based on
the data herein presented, it could also be stated that the type II calcifications
contain externally and internally trapped material inside crystals” (Figure 4, Figure 5).
6. CONCLUSION
Concluded is that
the data presented in this document supports a proposed mechanism whereby
Hydroxyapatite crystallization in malignant type II ductal breast carcinoma
tissue induces a backwards suction resulting from the piercing of malignant
cells, causing the shedding of protein and biological impurities into the
intercellular space. This material could then be transported
by the lymphatic system with its consequences, including additional kidney
failure Castellanos et al. (2008).
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
The author acknowledges the contribution of Mrs. Laura Embí Sánchez in proofreading the manuscript.
REFERENCES
B. N. Figgis, Malcolm Gerloch, Ronald Mason, and Ronald Sydney (1969). Nyholm the Crystallography and Paramagnetic Anisotropy of Potassium Ferricyanide. https://doi.org/10.1098/rspa.1969.0031.
B. Viswanath, R. Raghavan, U. Ramamurty, N. Ravishankar. (2007). Mechanical Properties and Anisotropy in Hydroxyapatite Single Crystals. Scripta Materialia, 57(4), 361–364. https://doi.org/10.1016/j.scriptamat.2007.04.027.
Castellanos,
M. R., Paramanathan, K., El-Sayegh, S., Forte, F., Buchbinder, S., &
Kleiner, M. (2008). Breast Cancer Screening in Women with Chronic Kidney
Disease: The Unrecognized Effects of Metastatic Soft-Tissue Calcification.
Nature Clinical Practice. Nephrology, 4(6), 337–341.
https://doi.org/10.1038/ncpneph0804.
Cox, R. F., & Morgan, M. P. (2013). Microcalcifications in
Breast Cancer. Lessons from Physiological Mineralization. Bone, 53(2),
437–450., PubMed: 23334083, https://doi.org/10.1016/j.bone.2013.01.013.
Embi BS, A. A. (2022). Introducing Hydrocolloid
Wound Dressing Energy Disrupting Human Tissue Metabolism. International Journal
of Research -GRANTHAALAYAH, 10(10), 58–65. https://doi.org/10.29121/Granthaalayah.v10.i10.2022.4836.
Embi, A. A. (2022). Introducing Methodology to Detect
Dead Tissue Stored Energy. International Journal of Research -GRANTHAALAYAH,
10(8), 20–29. https://doi.org/10.29121/granthaalayah.v10.i8.2022.4733.
Embi, A. A. (2023). Introducing Electromagnetic
Energy from Hydrocolloid Wound Dressing Paste Penetrating a Glass Barrier
Disrupting Human Skin Lipid Droplets Size and Membranes: Possible Implications
in Cancer Cells Genesis and/or Cure. International Journal of Research
-GRANTHAALAYAH, 11(2), 47–54. https://doi.org/10.29121/Granthaalayah.v11.i2.2023.5032.
Embí, A. A. BS MBA. (2020). Introducing Crystallization
Backward Suction Trapping Lipids and Debris as Proposed Additional Factor in
the Genesis of Coronary Artery Disease. International Journal of Research
-GRANTHAALAYAH, 8(9), 215–233. https://doi.org/10.29121/granthaalayah.v8.i9.2020.1174.
Gosling, S., Scott, R., Greenwood, C., Bouzy, P.,
Nallala, J., Lyburn, I. D., Stone, N., & Rogers, K. (2019).
Calcification Microstructure Reflects Breast Tissue Microenvironment. Journal
of Mammary Gland Biology and Neoplasia, 24(4), 333–342.
https://doi.org/10.1007/s10911-019-09441-3 Dec. Epub 2019 Dec 5, 333–342.,
PubMed: 31807966, PubMed Central: PMC690855.
https://doi.org/10.1007/s10911-019-09441-3.
Haka, A. S., Shafer-Peltier, K. E., Fitzmaurice, M.,
Crowe, J., Dasari, R. R., & Feld, M. S. (2002, September 15).
Identifying Microcalcifications in Benign and Malignant Breast Lesions by
Probing Differences in Their Chemical Composition Using Raman Spectroscopy.
Cancer Research, 62(18), 5375–5380. https://pubmed.ncbi.nlm.nih.gov/12235010/.
He, F., Springer, N. L., Whitman, M. A., Pathi, S. P.,
Lee, Y., Mohanan, S., Marcott, S., Chiou, A. E., Blank, B. S., Iyengar, N.,
Morris, P. G., Jochelson, M., Hudis, C. A., Shah, P., Kunitake, J. A. M. R.,
Estroff, L. A., Lammerding, J., & Fischbach, C. (2019). Dec.
Hydroxyapatite Mineral Enhances Malignant Potential in a Tissue-Engineered
Model of Ductal Carcinoma in Situ (DCIS). Biomaterials, 224, 119489. Epub
September 11, 2019. https://doi.org/10.1016/j.biomaterials.2019.119489.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2023. All Rights Reserved.