
Cell-penetrating Peptides Nano-conjugated with Metallic Nanoparticle for the Development of Therapeutic and or Prophylactic Agents Against Respiratory Syncytial Virus
1 Ph.D.
Student, Microbiology Program, Department of Biological Sciences, Alabama State
University, Montgomery, Alabama, USA
|
|
ABSTRACT |
||
|
Respiratory
Syncytial Virus (RSV) is an enveloped, pleomorphic, often filamentous,
cytoplasmic virus-containing non-segmented, negative-sense, single-stranded
RNA associated with viral proteins, making up a nucleocapsid core that is
enclosed within a lipid envelope. RSV causes about 7 % of deaths among
infants and young children globally, which is the second-most cause of mortality
in that age group after malaria. Despite the immense impact mounted by RSV in
public health and the economy, there are no effective prophylactic and
therapeutic agents to control and treat the disease caused by RSV. Currently,
four RSV vaccines and a monoclonal antibody candidate, all using the
stabilized pre-fusion (F) proteins, have shown promising results in healthy
subjects and are in phase III clinical trial. Results from these trials are
expected to be released soon. However, more than one type of vaccine and
therapeutics are required to cover all populations at risk: younger children,
older adults, pregnant women, and immunocompromised people. Search for more
antiviral drugs and vaccines is going on, but due to the issues of cost,
toxicity, resistance, bioavailability, and overall pharmacokinetic profile
associated with prospective traditional drugs, studies on antiviral peptides
can offer novel avenues in the field.
In recent years, cell-penetrating peptides (CPPs) with 5-30 AAs in
length have shown promising drug delivery potential, but antiviral property
demonstrated by some CPPs is another exciting possibility in the drug
discovery arena, since finding shorter anti-viral peptides is another
priority to minimize the cost. Some of the metallic nanoparticles have shown
antiviral properties themselves. If both cell-penetrating property and
antiviral activity can be found in the same peptide, nano-conjugating CPP
with or without other antiviral peptides can improve the stability and other
therapeutic indices of such peptide so that it can possibly be developed as
safe and effective therapeutic and or prophylactic tools to control RSV. |
|||
|
Received 25 June 2023 Accepted 26 July
2023 Published 09 August 2023 Corresponding Author Homa Nath
Sharma, hsharma1952@myasu.alasu.edu DOI 10.29121/granthaalayah.v11.i7.2023.5200 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2023 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Respiratory Syncytial Virus,
Cell-Penetrating Peptides, Metallic Nanoparticles |
|||
1. INTRODUCTION
Respiratory Syncytial Virus (RSV) is an enveloped, pleomorphic, often filamentous, cytoplasmic virus-containing non-segmented, negative-sense, single-stranded RNA associated with viral proteins, making up a nucleocapsid core that is enclosed within a lipid envelope (as depicted in Figure 1). It was named Respiratory Syncytial Virus due to its ability to produce a multinucleated mass of protoplasm formed by merging of cells in tissue culture cells, called syncytia Ruuskanen, O., & Ogra (1993), Shilovskiy et al. (2019), Battles & McLellan (2019). Human RSV (hRSV) belongs to the Paramyxoviridae family (which also includes measles, parainfluenza 1-3, Sendai, Hendra, and Nipah viruses under paramyxovirinae subfamily), Pneumovirinae subfamily (which also includes human metapneumovirus-hMPV, avian MPV-aMPV, pneumonia virus of mice-PVM, and ovine RSV-oRSV) and Monomegavirales order González et al. (2012), Kolli et al. (2012). Notable members of the genus, conventionally the pneumovirus, are human RSV (hRSV) and bovine RSV (bRSV) Kolli et al. (2012), Collins et al. (2013). Recently, hRSV has been reclassified into the Orthopneumovirus genus within the Pneumoviridae family Soto et al. (2020). hRSV has two antigenic subgroups: A and B, based on the variations or genome-wide sequence divergence corresponding to the large Glycoprotein (G), Fusion protein (F), Nucleoprotein (N), and Phosphoprotein (P) Ruuskanen & Ogra (1993). hRSV causes severe diseases of both upper and lower respiratory tract, including bronchiolitis and pneumonia, especially among the immune-compromised patients, newborns, young kids, and patients with chronic obstructive pulmonary disease or bronchial asthma, which can collectively be called as high-risk populations for hRSV Collins et al. (2013), Jartti & Gern (2017).
Figure 1
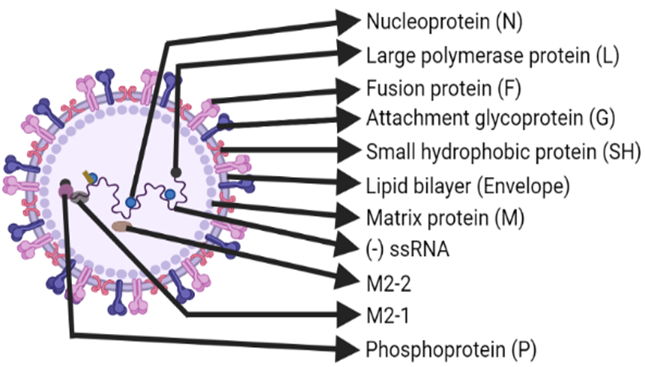
|
Figure 1 Structure of RSV. Created with BioRender.com |
hRSV virions produced in cell culture are predominantly
long filaments 60–200 nm in diameter and up to 10 μm in length.
However, spherical particles of 100–350 nm in diameter can also be seen,
while unstable infectivity as well as inefficient replication and budding in
vitro are the peculiar features of RSV Collins
et al. (2013) Graham (2013). The genome of RSV
contains 15,222 nucleotides (![]() .
In RSV, the following ten genes:
non-structural protein 1 (NS1), NS2, N, P (co-factor for polymerase),
Matrix protein (M), Small hydrophobic protein (SH), G, F, M2, and large
Polymerase subunit (L, catalytic polymerase enzyme domain) are encoded by the
genome (see Figure 2 for detail), and these
are transcribed as individual mRNA molecules into individual proteins, except
for the M2 gene mRNA, which has two overlapping open reading frames (ORFs) for
M2-1 and M2-2 proteins. That’s why a total of 11, not just 10, proteins are
encoded by RSV genome Shilovskiy et al. (2019), Collins
et al. (2013) (Figure 2).
.
In RSV, the following ten genes:
non-structural protein 1 (NS1), NS2, N, P (co-factor for polymerase),
Matrix protein (M), Small hydrophobic protein (SH), G, F, M2, and large
Polymerase subunit (L, catalytic polymerase enzyme domain) are encoded by the
genome (see Figure 2 for detail), and these
are transcribed as individual mRNA molecules into individual proteins, except
for the M2 gene mRNA, which has two overlapping open reading frames (ORFs) for
M2-1 and M2-2 proteins. That’s why a total of 11, not just 10, proteins are
encoded by RSV genome Shilovskiy et al. (2019), Collins
et al. (2013) (Figure 2).
Figure 2
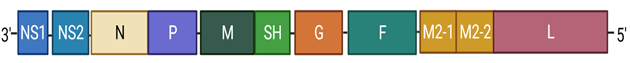
|
Figure 2 Genetic Sequence of RSV. Length of Rectangles Represent Approximate, But Not Exact Length of Specific Genes. Created with Biorender.Com |
The disulfide-bonded F- and G-glycoproteins are surface
proteins that act as antigenic determinants by eliciting protective and
neutralizing antibodies against RSV. The G protein facilitates viral attachment
and F protein facilitates syncytium formation in addition to viral penetration Ruuskanen & Ogra (1993). Of those two
major immunogen glycoproteins associated with the viral envelope, the G protein
appears to be less vital than F-protein for the infectivity process as the F-
protein, which is more conserved than the G protein, alone can also enable the
RSV to fuse with host cell Welliver (2003).
The non-globular, heavily glycosylated, and highly variable G protein bears a
CX3C fractalkine-like motif that modifies the cellular immune response. While G
protein is also expressed in the membrane-bound form, secreted form interferes
with antibody-mediated neutralization and interacts directly with
antigen-presenting cells to modify their function. The F-protein precursor is
activated by cleavage at two furin-cleavage sites
(KKRKRR’F-137 and RARR’E-110) by endoprotease into
disulfide-linked subunits: NH2-F2–F1-COOH Collins
et al. (2013). The sialylated F-protein activates the TLR-4 signalling
pathway, but the G-protein inhibits this. The SH protein is a viroporin (that forms a pentameric ion channel, also
supposed to delay apoptosis), but the function is not confirmed. The
non-glycosylated M protein in the inner envelope face helps in the virion
assembly by associating with F protein. Overlapping ORFs in the M2 mRNA encode M2-1
(in nucleocapsid), a transcription processivity factor, that attaches M protein
with ribonucleoprotein and M2-2 (regulatory protein) that shifts viral RNA
transcription to RNA replication Collins
et al. (2013), Ogra (2004). From another
overlapping set of L and M2 gene, M2 is expressed by
backtracking mechanism Collins
et al. (2013). Besides other
structural proteins discussed above whether the M2-2 is also packaged within
the virion remains to be determined González et al. (2012). As NS1 and NS2 are found only in infected
cells, but not in the virions, they are considered non-structural proteins Ruuskanen & Ogra (1993). They inhibit both
type I Interferon response (induction and signalling) and apoptosis Collins
et al. (2013). A 44-nucleotides (nt) extragenic leader
region before NS1 and 155-nt trailer region after L gene are found in the 3’
and 5’-end of the RSV genome, respectively.
A highly conserved 9-nt gene-start (GS) signal and moderately conserved
12–14-nt gene-end (GE) signal that ends with 4–7 U residues that encode the polyA tail by polymerase stuttering are found in each gene Collins
et al. (2013).
RSV causes about 7 % of deaths among infants and young children globally, which is second-most cause of mortality in that age group after malaria Morris et al. (2019). RSV causes yearly in-hospital death of about 60,000 children younger than 5 years of age, worldwide Battles & McLellan (2019), Shi et al. (2017). Battles & McLellan (2019). In the U.S. alone, yearly outpatient visits by 2.1 million of children younger than 5 years of age reflects the magnitude of situation. Average yearly hospitalization of 58,000 and 177,000 respectively and the average yearly death of 100-500 and 14,000 respectively in the children younger than 5 years of age and adults 65 years and older in the U.S. indicates the health and social burden created by the RSV. In the U.S. and similar areas in the world. RSV infections occur during fall, spring, and winter Centers for Disease Control and Prevention (US). (2020).
In cell culture, RSV viral antigen can be detected in nine hours and infectious viral particle can be obtained in 11-13 hours Ogra (2004). Due to the non-reversible deformation of F surface protein from pre to post trigger conformation, RSV undergoes quick inactivation losing infectivity in the cell. That’s why it is considered as unstable virus and is relatively difficult to grow in cell Beauchemin et al. (2019). The fragility of the virus can also likely be conferred by the long filamentous shape of the particle Collins et al. (2013).
The current knowledge on RSV infections and pathogenesis is generated by in vitro studies based on non-polarized epithelial cell line cultured on plastic. But this model, along with in vivo animal models which are semi-permissive to RSV infection, doesn’t reproduce natural replication, and inflammatory responses to be induced in human columnar airway epithelium in vivo Pickles (2013). As a feasible, scalable, reproducible, and affordable alternative, primary human airway epithelial culture (HAEC) can potentially be the appropriate in vitro model. It can offer advantage in studying host-pathogen interaction such as virus tropism and receptor preference. But for understanding of the intricate RSV-human interaction and immune reactions, airway organoids (AO) which is emerging as a promising three-dimensional (3D) culture can be a better option Rijsbergen et al. (2021). As the animal models, mice, cotton rat, chimpanzee, guinea pig and ferret are usually used to grow and study hRSV Ogra (2004). Among them, only chimpanzee is the animal permissive to hRSV replication and animal-animal transmission that reliably recapitulate at least the upper respiratory tract infection (URTI). Cotton rat and mice are used for negative selection of candidate vaccine, but their efficacy results may not be fully reflective to that of human. In majority of animal models listed above, natural progression of URTI to lower respiratory tract infection (LRTI) upon exposure to small dose of virus is not recapitulated. Instead, they require direct introduction of large dose of virus into lungs. Though resemblance of hRSV infection in children and bRSV infection in calves along with the antigenic similarities and genetic relatedness between hRSV and bRSV suggests non-human (calf)-pneumovirus animal model as an alternative, reproducible LRTI is supposed to be produced only by direct delivery of virus into lungs. Search for better animal model is continuous but higher animal species including chimpanzee and other non-human primates such as monkey can probably be better suited for pre-clinical studies of new therapeutic and prophylactic candidates Taylor (2017).
Despite the wide-ranging studies of clinical manifestations, diagnostic techniques, epidemiology, animal, and cellular models and the immunobiology of RSV, development of safe and effective prophylactic vaccine and antiviral therapeutic drug still remains the challenge Borchers & Gershwin (2013). In such scenario, a thorough understanding of host-pathogen interactions can define the targets for development of novel drugs and vaccines Russell et al. (2017). Nanotechnology is a fast-growing field in nanomedicine that aims at expanding the applications of various types of nanomaterials in diagnostic devices, pharmaceuticals, and the drug delivery system Morris et al. (2019). Similarly, application of natural or synthetic peptides can be one of the relevant approaches for the development of antiviral agents Shilovskiy et al. (2019). Antiviral peptides can be conjugated or encapsulated with nanomaterials for stability. In addition, antiviral peptides can be conjugated with cell penetrating peptides (CPP) for efficient cellular uptake or penetration Chakravarty & Vora (2021), Sadeghian et al. (2022). Some CPPs derived from viruses have shown both the characteristics of cell transduction and antiviral activities against same or different viruses Bultmann, H., Teuton & Brandt (2007), Akkarawongsa & Brandt (2008). This review will focus on the development of convincing antiviral drug based on nano-conjugated CPPs with or without incorporating another antiviral peptide in the light of RSV biology and immunology by conducting systematic literature review of the 130 PubMed-indexed articles, website contents and book chapters.
2. Replication of RSV
The RSV life cycle begins with the attachment of virion to
the apical surface of polarized, ciliated airway epithelial cells of host
through viral G protein Zhang
et al. (2002). RSV can use multiple
cell components as the cell receptors to invade the target cell. For example,
viral G protein which is a ![]() 300
amino acids (AAs) long type 2 single pass integral membrane protein, can bind
to the chemokine receptor CX3CR1 expressed on the apical region of bronchial
ciliary epithelial cells. RSV has also been found to attach to the immortalized
linear cells having the high density of heparan sulfate
proteoglycan (HSPG). Surface protein A (SP-A) and Annexin II as the cellular
receptors have also been suggested to be bound by G protein for initiating the
viral attachment Joshi
et al. (2019). Similarly, viral
entry can be ensured by binding of F protein with intercellular adhesion
molecule 1 (ICAM-1), epidermal growth factor receptor (EGFR) or nucleolin (100 kDa protein) of
cell membrane Shilovskiy et al. (2019), Battles
& McLellan (2019), Sun
et al.(2013). The 574 AAs long F
protein is a trimeric class I fusion protein that undergoes a conformational
change to bring about fusion of viral and host cell membrane. Entry of RSV by clathrin-mediated endocytosis or macropinocytosis
but without requirement of endosomal acidification has also been reported Kolokoltsov et al. (2007), Srinivasakumar et al. (1991).
300
amino acids (AAs) long type 2 single pass integral membrane protein, can bind
to the chemokine receptor CX3CR1 expressed on the apical region of bronchial
ciliary epithelial cells. RSV has also been found to attach to the immortalized
linear cells having the high density of heparan sulfate
proteoglycan (HSPG). Surface protein A (SP-A) and Annexin II as the cellular
receptors have also been suggested to be bound by G protein for initiating the
viral attachment Joshi
et al. (2019). Similarly, viral
entry can be ensured by binding of F protein with intercellular adhesion
molecule 1 (ICAM-1), epidermal growth factor receptor (EGFR) or nucleolin (100 kDa protein) of
cell membrane Shilovskiy et al. (2019), Battles
& McLellan (2019), Sun
et al.(2013). The 574 AAs long F
protein is a trimeric class I fusion protein that undergoes a conformational
change to bring about fusion of viral and host cell membrane. Entry of RSV by clathrin-mediated endocytosis or macropinocytosis
but without requirement of endosomal acidification has also been reported Kolokoltsov et al. (2007), Srinivasakumar et al. (1991).
This process is followed by the release of helical ribonucleoprotein (RNP) complex into the cytoplasm. Cytoplasmic viral inclusion bodies are supposed to concentrate viral products in the cytoplasm, where the viral RNA dependent RNA polymerase (RdRp) complex transcribes viral mRNA from virion RNA, synthesizes positive-sense anti-genome RNA intermediates needed for replication of new negative-sense RNA genomes for being packaged into the virions and caps (with methylation) and polyadenylates viral mRNAs Battles & McLellan (2019). The basic process of replication and stages in life cycle of RSV have been illustrated in Figure 3. The mRNA acts as a template for the translation into proteins. The translation is followed by modifications. The genome and antigenome lack 5’-caps or 3’- polyA tails and are bound separately for their entire length by the N protein to form stable nucleocapsids which remains intact throughout replication cycle and the virion so that the encapsidation protects RNA from degradation and shield from being recognized by host cell pattern recognition receptors (PRRs) Collins et al. (2013). Studies with mini-replicons show that N, P, L and M2-1 are the viral proteins required for the fully processive transcription, but M2-1 protein is dispensable for the RNA replication Collins et al. (2013), Collins et al. (1996).
Figure 3
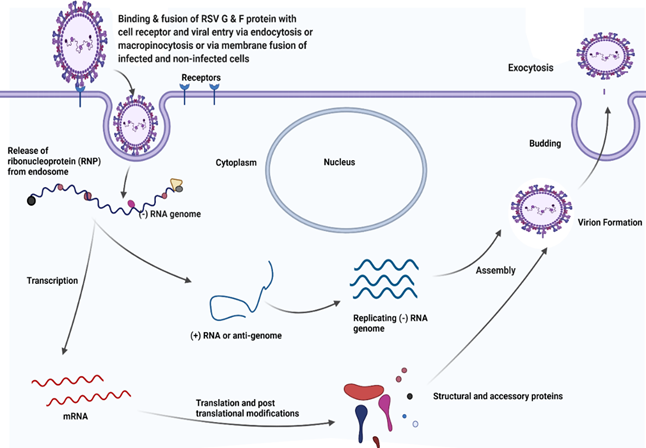
|
Figure 3 Replication Cycle of RSV. Created with Biorender.Com |
For the assembly of RSV virions, F proteins associate with lipid raft at or near the plasma membrane and then recruit M protein which precedes the actin-dependent outward membrane deformation facilitating the filament budding. Some studies support this model with some critical modifications like the ideas that filament formation initially occurs intracellularly and vesicle containing viral proteins traffic along microtubule before growing into filament and merging with nucleocapsid Battles & McLellan (2019). Four viral proteins: F, M, N and P are indispensable for the formation of virus like particles (VLP) that can transport viral genome into the target cells and viral filaments are formed only after the expression of those proteins Utley et al. (2008), Teng & Collins (1998). But with the possible dissociation of M layer from the viral membrane, F-protein may prematurely convert from prefusion to post fusion conformation so that the resulting non-filamentous, spherical, or pleomorphic viral particles may be less infectious Battles & McLellan (2019).
3. RSV immunopathogenesis
Airborne transmission by contact with epithelium of
nostrils, mouth, eyes, and skin of RSV-infected persons or by contact with a
surface contaminated with the RSV are the major ways of acquiring RSV Collins & Graham (2008), Carvajal et al. (2019). After entering the
airway epithelial cells (AEC) of the upper respiratory tract, downward
movement/transmission of virus into the lower respiratory tract leads to the
bronchiolitis (inflammation of bronchioles in the small airways) and or
pneumoniae (inflammation of alveolar space or air sacs of the lungs). While
bronchiolitis is characterized by wheezing, dyspnea, tachypnea, fatigue, fever and cough, pneumonia in the
children is manifested with fever, chest pain, wheezing, nausea, chills, and
other breathing difficulties Carvajal et al. (2019), Pickles & DeVincenzo (2015). During the
primary RSV infection, professional antigen presenting cells, mainly the
dendritic cells migrate to the lungs and pulmonary CD8+ T cell response is
supposed to be induced to ensure the viral clearance from the respiratory
system. However, systemic T-cell lymphopenia may precede the process. During
the reinfection, antibody generation in-terms of RSV specific IgG and IgA
mediated by B cell stimulating factor produced by the airway epithelium can be
protective even though humoral immunity in general is considered incomplete in
RSV infection. The nasal/mucosal IgA provides more protection during recovery
and shows the more durability than serum neutralizing antibodies Russell et al. (2017). An IgE response generated also against the RSV G and F
glycoproteins may impart a deleterious role, but the exact role of IgM that is
highly produced during primary RSV infection, is dubious Russi
et al. (1993). The generation
of Th1 cytokines, especially the gamma-interferon (IFN-γ) is strongly
correlated with protection. On the other side, Th2 biased inflammatory response
may be deleterious and neutrophilic inflammation mediated by Interleukin-8
(IL-8) is considered harmful response. In the borderline, local innate immune
factor including cathelicidin and IFN-λ,
cytokine like IL-17A and chemokine such as CCL-3 and CCL-5 may also play their
important roles in the pathogenesis Borchers et al. (2013), Russell et al. (2017).
Ciliated cells in the bronchial epithelia, type 1 pneumocytes
in the alveolus, intraepithelial dendritic cells (DCs) and basal epithelial
cells of the conductive airways, are the major cells reported as the target
cells for RSV infection Carvajal et al. (2019), Persson et al. (2014). During the
infection, the rounding, extrusion, and detachment of ciliated cells from the
apical zone of large airway epithelium induced by the viral NS2 protein results
into the accumulation of the infected cells in the airways lumen. The infection
of basal cells leads to the goblet cell proliferation, subsequent higher
production of mucus and increased influx of eosinophil. Additionally, massive
number of neutrophil (neutrophilia) releases neutrophil extracellular traps
(NETs). Accumulation of all those components and their movement into the distal
airways causes bronchial obstruction of narrow ducts and collapse of alveoli
causing acute inflammation and pathology in the lungs Carvajal et al. (2019),
Brinkmann et al. (2004),
Qiao et al. (2011).
Otitis media featured by middle ear effusion and ear pain, apnea, rhinorrhea, nasal
congestion, pharyngitis, cough, and crouch are also the clinical sequalae associated with
RSV infection González et al. (2012), Ogra (2004). While human
blood mononuclear cells and human alveolar macrophage, in addition to
respiratory epithelium are generally infected by RSV in immunocompetent
persons, RSV has also been recovered from liver, spleen, brain and myocardium
(heart) reflecting extra-pulmonary pathology in some immunocompromised patients
Ruuskanen & Ogra (1993). Suppression of
adaptive immune system, that would otherwise clear RSV from the host is the
major strategy of virus to invade various organs/sites of the body Carvajal et al. (2019). As RSV
infection desensitizes the alveolar macrophage to the TLR ligands and
upregulates the expression of inhibitory surface molecules, like PD-L1,
impairing T-Cell activation, subsequent viral or bacterial infections can be
promoted by RSV infections Didierlaurent et al. (2008), Telcian et al. (2011).
Immature immune and respiratory systems in infants (<6
months) pronounced highly in case of premature delivery (<35 weeks of
gestation) and malnutrition, low lymphocyte level in patients under 5 years old
and low level of RSV-specific neutralizing antibodies in the adults over 65
years are host associated risk factors for RSV infections Hall et al. (1979), Perk & Özdil (2018), Okiro et al. (2008), Welliver (2003). Male infants
are more likely to have acute bronchial obstruction as their airways have
smaller diameter than that of female infants Glezen et al. (1981), Ramos-Fernández et al. (2017). Similarly,
severe RSV disease is found to be associated with strong neutrophil response
along with the increased concentration of proinflammatory cytokines such as
interleukin-6 (IL-6), IL-8, Tissue necrosis factor-alpha (TNF-α) and
thymic stromal lympho protein (TSLP). Reduction of
RSV-specific memory CD8+ T cells over the age in older adults is another factor
for severity of infections Carvajal et al. (2019).
4. Drugs and vaccines
Supportive
management of clinical symptoms of RSV infections by providing
antibiotics to minimize the risk of bacterial infections, drugs (like α
and β adrenergic agonist as the bronchodilator) to reduce the adverse
effect of inflammation, and the
rehydration and oxygen therapy are the only two viable options available now for the general population Joshi
et al. (2019), Simões
et al. (2018). While ignoring the conflicting success of RSV
treatment with broad-spectrum drug ribavirin that can be applied
non-specifically against various RNA viruses, its use is still constrained with
its adverse side-effects Simões
et al. (2018), Janai et al. (1990). Prophylaxis of RSV in high-risk
infants, but still requiring more evidence of usefulness in cystic fibrosis
cases, with monoclonal antibody (mAb) Palivizumab
remains only one drug licensed and available in the market Simões
et al. (2018).Use of this and other mAbs
is also associated with the issues of high price and efficacy Wang
et al. (2008). Nirsevimab, another mAb, against properly folded preF
protein, which was stabilized by adding a chemical bond so that double-sided
tape-like shape exposes its important targets for neutralizing antibodies, has
shown efficacy at controlling RSV infections in full-term and healthy pre-term
infants during the phase III clinical trial jointly run by Sanofi and Astrazeneca. Full results may take few years to come Powell
(2021).
Regarding the vaccine, due to the unfortunate consequence
with the first formalin-inactivated RSV (FI-RSV) vaccine which caused
complications which is called vaccine enhanced disease and even death in some
vaccinated RSV-naïve children after subsequent RSV infection, scientists and
researchers should have been highly cautious in testing other vaccine
candidates Higgins et al. (2016). As of December
28, 2021, 98 studies of various types of vaccine candidates against RSV are
registered in the ClinicalTrials database
(https://ClinicalTrials.gov/). Many such
candidate vaccines and potential treatments based on monoclonal antibody and
other strategies which have passed pre-clinical studies are likely to be
available in upcoming years Simões et al. (2018). Developing a safe and effective
vaccine candidate for pediatric populations
(specially <6 months of their life) is of higher priority Higgins et al. (2016). With the
lessons learnt from FI-RSV vaccines that ended up failing with the vaccine
enhanced disease in RSV-naïve infants, vector and subunit vaccines attenuated
in vivo have been projected to be safer and more suitable than other approaches
in infants. As the maternal immunization is also a permissible route for
childhood RSV prophylaxis, any vaccine or medication based on the subunits,
viral particles or nanomaterials can be promising to administer with compliance
to the safety standards for pregnant women so that immunity can be transferred
into babies Simões et al. (2018). As the
life-long immunity is not induced with natural RSV infection and the repeated
infections in once-infected person is often frequent, devising strategies to
elicit and maintain long-lasting immunity in people with any vaccine candidate
is the current challenge in the field Kamphuis et al. (2013). Currently, four
RSV vaccine candidates, all using the stabilized preF
Proteins, have shown promising results in healthy subjects and are in phase III
clinical trial. First two from Pfizer and GSK involves stabilized preF protein only. Third candidate from Jansen includes in
same shot the pure preF protein and modified Adenovirus
that also produces preF after getting inside body.
Fourth jab candidate contains genetically modified mRNA that generates preF protein after RNA gets inside the cells Powell (2021) . Results from these trials
are expected to release soon. However, more than one type of vaccines is
required to cover all populations at risk: younger children, older adults,
pregnant women, and immunocompromised people Powell (2021), Dudas & Karron (1998).
5. Cell-penetrating Peptides (CPP)
Plasma membrane is a cellular barrier with selective permeability that maintains the survival and vital functions of living cell. This barrier is traversed by small molecule drugs either by direct diffusion through the lipid bilayer or any other natural processes. Similarly, membrane mobile transport is the strategy used by protein-based drug to enter the cells. However, ensuring efficient passage of cargo through the cellular membrane continues to be a foremost challenge for intracellular delivery of exogenous bioactive molecules or drug. In this context, improving the efficiency of transport of therapeutic agents into cell remains to be of higher priority. The membrane perturbation and the viral vector methods which are currently available techniques for the delivery of macromolecule, are associated with low delivery yield, high immunogenicity, and toxicity Wang et al. (2014), Jiang et al. (2012), Milletti (2012).
CPPs, also called protein transduction domains (PTDs), are diverse groups of peptides, mostly positively charged, usually with 5-30 amino acids length, which are considered as a molecular transporter. They are unique to other peptides in a sense that they can cross the cellular membrane, so they are called cargo translocator Wang et al. (2014), Jiang et al. (2012), Milletti (2012), Derakhshankhah & Jafari (2018). CPPs are known for facilitating cellular uptake of functional peptides and functional motifs inside the cell. As vectors, CPPs carries nucleotides, plasmid DNA, siRNA, small molecules, nanoparticles, peptides, and proteins, both in vitro and in vivo Milletti (2012). The cargo can be incorporated or conjugated with CPPs either by covalent bonding for e.g., disulfide bond, amide bond or linker formed specially in the case of neutral cargo, or by non-covalent ones including electrostatic or hydrophobic interactions in case of negatively charged cargo. As different cargos need different process of covalent conjugation making first approach the time-consuming one, latter approach can be flexible enough for wide application in cargo delivery Derakhshankhah & Jafari (2018), Copolovici et al. (2014) . The electrostatic interaction of amphiphilic and Argininine-rich CPPs with negatively charged cell membrane may be one out of various mechanisms suggested for efficient delivery or internalization of cargo to be facilitated by CPPs Andaloussi et al. (2011). Although, CPPs demonstrate their potential role in pharmaceutical application in terms of their antiviral, antifungal or antibacterial properties, they are mainly used in drug delivery system and called Trojan horse as they can pass through the lipid bilayer of cell membranes carrying polar molecules, including large hydrophilic proteins with higher molecular weight De Oliveira et al. (2021). Due to the low cytotoxicity and higher transduction (internalization) efficiency of CPPs, they can be used for various biomedical applications both diagnostic and therapeutic ones such as transport of molecules into induced pluripotent stem cells for guiding differentiation, delivery of cargo including proteins and peptides for therapeutic application, and the delivery of radioactive compounds or fluorescent dye for imaging. CPPs such as anti-inflammatory peptide 6 (AIP6) has also been found to interact with p65, one of the subunits of NF-kB, displaying the anti-inflammatory effects. Trans-activator of transcription (TAT) derived from HIV-TAT protein and penetratin from Drosophila Antennapedia homeodomain were the first CPPs considered for their role in the cell penetrating property and intracellular cargo delivery Derakhshankhah & Jafari (2018), Kurrikoff et al. (2016), Wang et al. (2011).
Figure 4
|
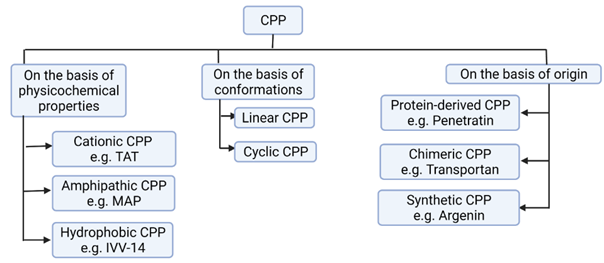
Figure 4 Types of CPP. CPP, Cell-Penetrating Peptide; TAT, Transactivator of Transcription; MAP, Model Amphipathic Peptide; IVV-14, In Vitro Virus-14. Classifications of CPP is Based on Milletti (2012), Xie et al. (2020). Created with BioRender.com. |
As depicted in Figure 4, based on the physicochemical properties or nature, CPPs can be classified into three types: Cationic, amphipathic, or hydrophobic. As TAT and penetratin involves polyarginine and lysine residues in their structure, they are cationic CPPs. Similarly, due to the high degree of amphipathicity (containing both water soluble, polar and water insoluble, non-polar portions) caused by Lysine residues in their primary sequences, Transportan, Pep-1 and model amphipathic peptides (MAP) are amphipathic CPPs. As the pept1, Pept2 and In Vitro Virus 14 (IVV-14) contain non-polar sequences or hydrophobic motifs, they are categorized as hydrophobic CPPs. Hydrophobic CPPs can be further classified into linear hydrophobic peptides made of natural amino acids or chemically modified CPPs which can be stapled (closed in ring), prenylated or pepducins one (made by N-terminal lipidation). Due to the inherent ability of signal peptides to facilitate the interaction of nascent protein into specific cell organelles, these are considered as the rich sources of hydrophobic CPPs. Amphipathic CPPs, which are mostly chimeric, or sometimes proteins-derived, can also be categorized into primary, secondary Alpha-helix, secondary Beta-sheet or Proline-rich ones Milletti (2012), Derakhshankhah & Jafari (2018)Due to having positively charged residues such as Arginine and Lysine, most of the studied CPPs are cationic Milletti (2012), Derakhshankhah & Jafari (2018). The most positive charge of cationic CPP has higher affinity with negatively charged glycoprotein of cytoplasmic membrane. The cationic residues especially the poly-arginine stretches have been proven to confer highest cell uptake and better therapeutic index Xie et al. (2020). In addition to containing stretches of positive charges, three-dimensional (3D) arrangements of cationic CPPs should not form amphipathic helix or show hydrophobic characters. However, both cationic and hydrophobic CPPs may have overlapping net positive charge close to +0.2, while amphipathic and hydrophobic CPPs may have net negative charge implying the fact that both can be either anionic or cationic Milletti (2012). Increased number of arginine residues in general increases the cellular uptake level. In contrast, polylysine level is considered to decrease the cellular uptake level. In the case of arginine-based peptides, octaarginine is the minimal sequence for efficient uptake. However, the nuclear localization signal with less than eight positive charges can be conjugated to hydrophobic peptide to get amphipathic CPPs having improved uptake profile. Milletti (2012), Tünnemann et al. (2008). Even if most amphipathic CPPs are cationic, function of amphiphilicity, not charge is responsible for membrane translocation efficiency of those CPPs. As the uptake of amphipathic CPPs can be easily reduced by even a single point mutation, amphipathic CPPs in general are not proper CPPs Milletti (2012). Furthermore, any change in secondary or 3D structure including the interruption of di-sulfide bonds required to maintain helical conformation of amphipathic CPP lactoferrin or the D-mutations abolishing the beta-sheet formation in VT5 peptide have been associated with significantly reduced uptake by cell Duchardt et al. (2009), Oehlke et al. (1997). But as the most cationic CPPs are likely to form coiled conformation, their 3D structural change might not impact the cellular intake in significant way. Also, in hydrophobic CPPs, amino acid composition, not the conformation has been found to modulate cellular intake Milletti (2012).
CPPs can also be classified into protein-derived, chimeric, and synthetic ones based on origin (Figure 4). As penetratin and TAT are derived from natural proteins, they are protein derived CPPs. As Transportan is composed of two or more motifs from different peptides: galanin and mastoparan, it is considered as chimeric CPP. Similarly, as the Argenin is the only structural component to be mimicked by polyarginine family, this is synthetic CPP Derakhshankhah & Jafari (2018), Bechara & Sagan (2013).
Figure 5
|
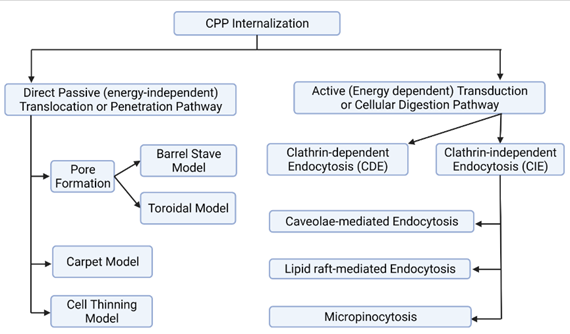
Figure 5 Potential Pathways of CPP Internalization. Detailed Description is in the Preceding Texts. Created With Biorender.Com |
The pathway selection for the CPP internalization into the cellular membrane has been found to be dependent mainly on the three parameters namely: peptide sequence, peptide concentration and lipid components in each membrane Derakhshankhah & Jafari (2018). On the basis of peptide sequence, uptake mechanism of amphipathic CPPs like MAPs whose membrane transduction rely on helical amphipathicity and a length of at least four complete helical turns, could be differed from that of Tat and penetratin analogs that depend on enhanced local concentration of positive charge caused by electrostatic interaction of arginine and lysine in cationic CPPs Bellet-Amalric et al. (2000), Ziegler et al. (2003), Oehlke et al. (1998). Similarly higher peptide concentration may Favor rapid cytosolic uptake via direct penetration and lower concentration may induce endocytosis mediated uptake mechanism of cationic CPPs Duchardt et al. (2009), Padari et al. (2010). The concentration threshold which is generally supposed to be in low micromolar range, may depend on both type of CPP and type of cell or its membrane composition Jiao et al. (2009). As the electrostatic interaction of positive charge of CPPs with biomembrane is mediated by the negative charge in membrane conferred by heparin sulphate proteoglycan and phospholipid, lipid component of biomembrane is also a factor determining the mode of uptake of CPPs Silhol et al. (2002), Mann & Frankel (1991) .
After formation of electrostatic interaction of CPPs with cell surface glycosaminoglycans (GAGs), clustering of GAGs at the cell surface, activation of intracellular signals, and actin remodelling with membrane curvature are triggered sequentially. Then comes the cell entry through different internalization pathways, which are summarized in Figure 5. In direct translocation of cationic CPPs, acid sphingomyelinase activation followed by a change in the composition of the lipid of the cell membrane may mediate the further process Verdurmen et al. (2010). Direct passive or energy independent translocation which occurs at higher CPP concentration, is suggested to occur in primary amphipathic CPPs like Transportan and AMP, preferably at low temperature. This single-step process involves the formation of inverted micelle (transitory structure), pores or carpet model Ruseska & Zimmer (2020), Madani et al. (2011). Inverted micelle model is compatible with the translocation of small hydrophilic peptides in which interaction of hydrophobic residues like tryptophan of CPPs with the hydrophobic part of the membrane, in addition to the usual electrostatic interaction between the CPP and components of the lipid membrane, is involved. Inverted micelle is formed after the disruption of micelle bilayer membrane and this mechanism involves three steps. First, CPPs are trapped into hydrophilic environment of micelle core. Second, inverted micelle is destabilized after its interaction with membrane components. Third, CPPs can then be released into cytoplasm Derakhshankhah & Jafari (2018), Derossi et al. (1998), Elmquist et al. (2006).
Pore formation may occur through two modes: the barrel stave and the toroidal ones. In the barrel stave mode, barrel is created by helical CPPs through hydrophobic residues, found adjacent to the lipid chains, and central pore is formed by the hydrophilic residues. In the toroidal model, lipids bend in such a manner that CPPs can always be pointed to the head group and pore is formed by both CPPs and lipids Thorén et al. (2003), Wadia et al. (2004). Carpeting and thinning of the membrane occur after the interactions between negatively charged phospholipid and cationic CPPs, but before the consequent internalization at higher than threshold concentration of CPPs, in carpet or membrane thinning model respectively Derakhshankhah & Jafari (2018), Pouny et al. (1992). Translocation through the formation of pore, carpet or cell thinning have been referred by some author as penetration mechanism Derakhshankhah & Jafari (2018).
Endocytosis mediated entry occurs by two mechanisms: Clathrin-dependent endocytosis (CDE) and Clathrin-independent endocytosis (CIE). In CDE, adaptor proteins recognize the cytoplasmic domains of plasma membrane proteins and package into Clathrin-coated vesicles that are for bringing them into the cell. CIE can proceed in the form of Caveolae and/or lipid raft-mediated endocytosis and micropinocytosis Mayor & Pagano (2007). Size may be critical factor for endocytosis mediated uptake. For example, less than 200 nm as the optimum size of CPP-cargo complex has been suggested for the efficient endocytic uptake Maeda (2010). Endocytosis or cellular digestion is an active, energy-dependent transduction approach in which plasma membrane folds inward so that materials are transported from the outside of cells and absorbed by the cell. Phagocytosis and pinocytosis are endocytosis involved with large particles and solutes respectively. In receptor-mediated endocytosis, Clathrin and Caveolin pits cover the intracellular part of cell membrane facilitating both invagination and attachment of extracellular molecule to membrane receptors. Then Clathrin-coated (larger) and Caveolin-coated (smaller) vesicles are formed Ter-Avetisyan et al. (2009). Endocytosis is the major cell uptake pathway for CPPs Mishra et al. (2011). While certain uptake mechanisms have been suggested for certain CPPs, more than one mechanism can work for the same CPP. For instance, a combination of both direct translocation and endocytosis can be attributed for the uptake of arginine-rich peptides Ruseska & Zimmer (2020).
Efficiency of delivery of the CPPs may depend on various
parameters including but not limited to the type of peptide sequence, nature of
CPPs and size of cargo-CPPs complex Derakhshankhah & Jafari (2018). Rational design of
CPPs should take into account the inclusion of key amino acids in specific
position, such as arginine for complexation with cargo, like nucleic acid, and
uptake by cell; histidine to enable escape from endosome; cysteine for
controlled cargo release and stability; and tryptophan to improve hydrophobic
interaction with cell membranes McErlean et al. (2021). For the CPPs
to be perfect, specific residues (e.g., Arg, Pro, Trp, Leu, Lys, and Ala) are desired at certain positions Gautam et al. (2013). However,
examples below support the notion that there is no universal rule regarding
types of AAs to be included in the CPP.
HIV-1 TAT= RKKRRQRRR (Cationic CPP (natural or not designed) Milletti (2012)
Transportan= GWTLNSAGYLLGKINLKALAALAKKIL (Amphipathic CPP) Pooga et al. (1998)
Pep-7= SDLWEMMMVSLACQY (Hydrophobic CPP) Gao et al. (2002)
VG-21=VTPHHVLVDEYTGEWVDSQFK Tiwari et al. (2014)
Many therapeutic agents can enter the cell and nucleus by CPP or CPP-conjugated molecules like the CPPs-functionalized nanoparticles without interruption of cell membranes. Efficient delivery of these peptides could both improve the bioavailability of drug/cargo and augment the efficiency of that therapeutics Derakhshankhah & Jafari (2018) . To be considered for any biomedical applications, cellular uptake profiling of CPPs is not enough. So, the CPPs, like small molecules, must be assessed for toxicity, tissue distribution, cell selectivity, solubility, and plasma stability Milletti (2012). The lack of oral bioavailability and short duration of action are still the problems of CPPs which are supposedly caused by physical and biochemical barriers for absorption created by gastrointestinal epithelium and enzymatic proteolysis before reaching the site of action respectively Milletti (2012), Renukuntla et al. (2013), Nestor (2009). Novel routes of administration including intranasal, and inhalation ones and injectable depot formulations are expected to solve the bioavailability issue. As proteolysis and rapid renal clearance are causing short duration of action, proteolysis can be prevented by using unnatural amino acids and stabilization of the 3D conformational structure, possibly with nano-conjugation, whereas using carrier protein and reducing the amount of free peptide in plasma by depot formation in injection site can address the renal clearance issue Milletti (2012), Nestor (2009).
6. Anti-RSV peptides
Though, the humanized monoclonal antibody-based drugs like Palivizumab, IGIV and MEDI-524 have initially shown exciting usefulness for RSV prevention, they are likely to be constrained by their possibly higher production cost and pricing in the market Wang et al. (2008). Some organic compounds with low molecular weight, can bind F protein and prevent virion internalization by inhibiting virus fusion, but they have also proven ineffective over the time as the mutations in the F protein has conferred resistance against those fusion inhibitors in RSV variants Battles et al. (2016). These two limitations signify the urgent need for developing new approaches in designing anti-RSV agent. Natural peptides like Cathelicidins and Defensins (both cationic ones), and their synthetic analogs have already shown their antiviral activity against HIV-1, human papilloma virus, herpes simplex virus, influenza virus, and the hepatitis B and C viruses demonstrated both in vitro and in vivo experiments Gwyer Findlay et al. (2013). Given the similarities in many respects of RSV with above mentioned viruses, for instance the fusogenic nature of both RSV (F protein) and HIV-1 (gp41), development of anti-RSV peptides is promising Shilovskiy et al. (2019). Antiviral activity against whole range of pathogens (broad spectrum activity) and multitudes of mechanism of action ensuring to target many different stages of virus life cycle is the main merit of antiviral peptides. Biocompatibility and the low toxicity are additional advantages Fjell et al. (2011). Some anti-RSV peptides have been listed in the Table 1 below.
Table 1
|
Table 1 Notable Anti-RSV Peptides |
|||
|
S. N. |
Peptides |
EC50, μM |
References |
|
1 |
Human Beta-Defensin 2 (HBD2, found in
human) |
0.46 |
Kota et al. (2008) |
|
2 |
T-118 (from Heptad Repeat region B-HRB
of RSV F-protein) |
0.051 |
Lambert et al. (1996) |
|
3 |
HR1-30a (from HRA of RSV F-protein) |
1.68 |
Wang et al. (2003) |
|
4 |
G149-177 (from RSV G-protein) |
4.0 |
Gorman et al. (2001) |
|
5 |
LL-37 (from Cathelicidin,
a natural protein) |
1.1-9.3 |
Currie et al.
(2013), Currie et al. (2016) |
|
6 |
CXCL-9 (74-103) (from human) |
31.0 |
Vanheule et al. (2016) |
|
EC50, μM=50
% micromolar concentration of peptides for virus inhibition |
|||
Anti-RSV peptides are supposed to block viral attachment and or fusion by targeting either viral G and F proteins or the host cell receptors. They can also act as virucidal agent by disrupting viral envelope. Since both mechanisms correlate to the initial stages of life cycle of RSV, antiviral peptides can be considered only for prophylaxis. However, finding space for improving immunoregulatory properties required for therapeutic use can be another avenues in the RSV field Shilovskiy et al. (2019). Though antiviral peptides are also suggested to bind cell receptor thereby altering the cell signalling pathway, to interfere the fusion of viral membrane with endosome of host cell, and to inhibit viral enzyme required for virus replication, further studies are needed to figure out the exact mechanisms Krajewski et al. (2004), Delcroix & Riley (2010). Another concern for anti-RSV peptides is their low resistance to the degradation from serine protease. To overcome this problem, the peptides can be applied in locally active inhalation forms given the relatively lower level of protease level in respiratory epithelium than in the systemic circulation. Other methods to stabilize the peptides may be worth finding Shilovskiy et al. (2019). While many peptides such as 35 AAs T-118 derived from heptad repeat B (HRB) domain of RSV F protein and 62 AA HR1-30a from HRA domain of RSV F protein were demonstrated to inhibit RSV in vitro Lambert et al. (1996), Wang et al. (2003), they are not going for clinical trials due to the higher production cost, low or no oral availability and low stability (less half-life) in the blood circulation Sun et al. (2013). Development of shorter peptide that can perfectly inhibit the RSV entry is more desirable goal but retaining the 3D structure and the antiviral activity of shortened peptide is the challenge that remain to be overcome Sun et al. (2013), Shepherd et al. (2006).
7. Nanocarriers or nanoparticle (NP)
Besides vaccinations, use of antiviral drugs is another tantamount method to control the burden of viral disease. A broad spectrum of natural substances including peptides, essential oils, and phenolic compounds like curcumin from turmeric, and synthetic drugs including siRNA, and ribavirin, have already been identified as antiviral agents Delshadi et al. (2021), Moghadamtousi et al. (2014). However, pure form of antivirals may be easily destroyed in GI tract or amount of antiviral being absorbed can be diminished. Low permeability through cell membrane or mucosa is another concern for the antivirals. Even if above two barriers are overcome, rapid metabolism and fast excretion from body system can still compromise the efficacy of the drug. Using novel and advanced drug delivery system can potentially solve these problems Delshadi et al. (2021).
Nanoparticles being small sized, can move more freely in the human body with fewer plasma fluctuations reducing the adverse effects. As the absorptive endocytosis is the typical uptake mechanism for nanodrug and nanosized structure can easily penetrate the tissue systems, higher bioavailability of drug obtained in this manner can also ensure the action of antiviral drugs at targeted locations. Slow or controlled release at specified dose along with stability of antiviral drug conjugated or encapsulated with nanoparticles in blood circulation for prolonged period are thus certain to augment the efficacy of the drug. Some drug delivery systems including the liposome and micelles containing inorganic metallic nanoparticles can protect the drug from being degraded in the gastrointestinal tract and can deliver even the sparingly water-soluble drug to their target locations Patra et al. (2018), Kabanov et al. (2002).
Various lipid-based (liposome, solid-lipid nanoparticle-SLN, nanoemulsion), polymer-based (hydrophilic chitosan, alginate etc; hydrophilic polylactic acid-PLA, polylactic-co-glycolic acid-PLGA etc; micelles, nanocapsule and nanosphere), carbon based (carbon nanotube-CNT, graphene, fullerene), inorganic-metal-based (Gold nanoparticle-AuNP, Gold nanorod-AuNR, Silver nanoparticle-AgNP etc), lipid-polymer-hybrid-based, surface modified and stimuli sensitive nanomaterials can be used for antiviral drug delivery Chakravarty & Vora (2021). Better surface functionalization and property of surface plasmon resonance have rendered AuNPs and AgNPs more biocompatible and versatile than other drug delivery vehicles including liposomes, micelles, and dendrimers. Some metallic nanoparticles have also shown antiviral properties themselves or enhanced the therapeutic index of incorporated drug Chakravarty & Vora (2021), Yadavalli & Shukla (2017). Due to their unique structure, size, shape, and local-field enhancement action, metal nanoparticles can easily bind with surface proteins of virus through van der Waals forces and Kazimir interaction causing its inactivation Chakravarty & Vora (2021). While AgNP as a cluster of colloidal form can be less toxic than ionic one, its unique physicochemical property such as larger surface area to volume ratio can generate efficient interaction with microbial proteins Morris et al. (2019). As an antiviral against RSV, AgNP can bind with sulfur-containing residue on surface glycoprotein and block the viral attachment and entry into the host cell Lara et al. (2010). In the second potential mechanism of action, AgNP can penetrate the cell membrane and block the host factors required for assembly of progeny virus Morris et al. (2019), Greulich et al. (2011). Gold nano rod (GNR) alone, was also found inhibitory against hRSV in Hep-2 cells and BALB/c mice by inducing innate immune response, but further mechanistic studies can elaborate on the detailed mechanism Bawage et al. (2016). The gold nanoparticle (AuNP) polycarboxylated (with carboxyl group polymer) via [(2-amino-ethoxy)-ethoxy]-acetic acid (AEEAc) and functionalized with anti-RSV peptides derived from HR region of RSV F-protein can synergistically bind to the F-protein of infective virus. This binding can block not only the virus into the host cell membrane, but also the subsequent fusion of infected cell with new healthy cells, thereby preventing the syncytia formation and subsequent inter-cellular transmission/infection Singh (2012). In another study, both AuNP and AgNP have been reported to increase both solubility and antiviral property of FluPep (an anti-influenza peptide), proving these nanoparticles as a perfect antiviral drug delivery system Alghrair et al. (2019). However, information regarding the issue of their toxicity and potential mechanisms of in vivo transport and uptake of metallic nanoparticle is not enough. Further well-designed studies are required to resolve the issues Patra et al. (2018).
8. Nano-conjugated CPP as a vehicle for antiviral peptide against hRSV
Figure 6
|
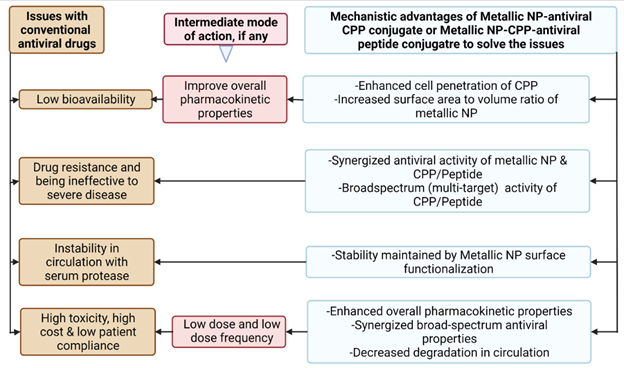
Figure 6 Potential Advantages of Anti-RSV Peptides Nano-Conjugated with CPP. Created with Biorender.Com |
The overall potential advantages of nano-conjugated
CPPs in terms of the mechanistic ease have been depicted in Figure 6. Though, antiviral
peptides can be better at specificity, potency, and low side effects as
compared to small molecule chemical agent, their therapeutic application can be
constrained by their low ability to traverse the cell membrane Sadeghian et al. (2022). Conjugation of these
peptides with CPP has not only ensured their successful delivery, but also
enhanced the therapeutic efficacy of peptides/proteins against the viruses both
in vitro and in vivo Mino et al. (2008), Chu et al. (2016).
To improve also the other pharmacokinetic properties of antiviral drug,
specifically the hydrophobic ones, drug can be loaded into the nanomaterials.
Reduced exposure of drug to the external environment, resulted by doing so, can
protect the drug from systemic degradation. Similarly, potentially minimized
cytotoxicity, prolonged circulation time, improved bioavailability and ensured
release into specific targeted sites can increase the therapeutic potential of
the drug Sadeghian et al. (2022). Additionally,
nanomaterials themselves can demonstrate the antiviral activity Sadeghian et al. (2022), Tang et al. (2021).
However, by virtue of their large size, spatial occupation, 3D structures and
undesirable hydrophilic/hydrophobic nature, their intracellular delivery as
well as cargo transport can sometimes be problematic Munyendo et al. (2012). But,
conjugation of nanocarrier with CPP has the potential to enhance the
intracellular delivery of cargo-loaded nanoparticle Desale et al. (2021). As the inactivation
of CPPs-cargo complex by proteases can compromise efficiency, selectivity and
capacity of cargo transport, incorporation of nanocarrier can be crucial to
stabilize the CPPs Chakravarty & Vora (2021), Reissmann (2014).
Additionally, dual delivery systems
that benefits from the merits of both CPPs and nanoparticle may ensure the more
precise delivery, improved performance, extended half-time life, more stability
and increased drug loading Silva et al. (2019). Suggested mechanism of action of
anti-RSV peptides nano conjugated with CPP has been illustrated in detail in Figure 4 below.
Similarly, as the covalent conjugation of any drug with
releasable hydrophilic polyethylene glycol (PEG) can avoid fast uptake by
reticuloendothelial system (RES) including macrophage in the body, PEGylation
of nanocarrier-peptide complex might be instrumental in reducing
immunogenicity. It can also improve drug solubility, stability and retention
time with minimized proteolysis and renal excretion/clearance thereby ensuring
optimal efficacy of antiviral drug even with reduced dosing Veronese & Mero (2008),
Otsuka et al. (2003).
In peptidomimetics, peptides derived from viral protein can
mimic binding property of viral protein to the specific host protein so that
protein-protein interaction required for the replication cycle of virus can be
disrupted, which is one of the mechanisms also reinforced by CPP Bultmann & Brandt (2002), Cochran (2000). Similarly, in
addition to being the promising cargo delivery vehicle, several CPPs derived
from one virus have displayed antiviral properties themselves against the same
or different viruses. In majority of these cases, the net positive charge of
peptides is supposed to block attachment or entry of virus into host cell by
interacting to the negatively charged host or viral components Bultmann et al. (2007), Keogan et al. (2012), Akkarawongsa et al. (2008). In the future,
designing the CPP with either peptidomimetic or optimal cationic charge that
can impart sufficient viral inhibition both in vitro and in vivo can
potentially give us an edge in the fight against RSV infections.
9. Conclusion
RSV is one of the major viral infections of the world affecting infants, younger children, elderly, and immunocompromised persons. Despite the immense impact mounted by RSV in public health and economy, there are no effective prophylactic and therapeutic agents to control and treat the disease caused by RSV. Currently, four RSV vaccines and a monoclonal antibody candidate, all using the stabilized pre-F proteins, have shown promising results in healthy subjects and are in phase III clinical trial. Results from these trials are expected to release soon. However, more than one type of vaccine and therapeutics are required to cover all populations at risk: younger children, older adults, pregnant women, and immunocompromised people. Search for antiviral drugs and vaccine is going on, but due to the issues of cost, toxicity, resistance, bioavailability, and overall pharmacokinetic profile associated with prospective traditional drugs, studies on antiviral peptides can offer novel avenue in the field. In recent years, CPP with 5-30 AAs in length have shown promising drug delivery potential, but antiviral properties demonstrated by some CPPs is another exciting possibility in the drug discovery arena, since finding shorter anti-viral peptide is another priority to minimize the cost. Some of the metallic nanoparticles have shown antiviral property themselves. If both cell-penetrating property and antiviral activity can be found in the same peptide, nano-conjugating CPP with or without antiviral peptides can improve the stability and other therapeutic indices of such peptide.
Author’s Contribution
HNS is the sole contributor of the work.
Author Information
HNS is working on multiple areas of infectious diseases including HIV/AIDS, SARS-CoV-2/COVID-19, RSV and Toxoplasma gondii.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
I am thankful to the Ph. D. Microbiology program at Department of Biological Sciences, Alabama State University, Montgomery, Alabama, USA.
REFERENCES
Akkarawongsa, R., Potocky, T. B., English, E. P., Gellman, S. H., & Brandt, C. R. (2008). Inhibition of Herpès Simplex Virus Type 1 Infection by Cationic Beta-Peptides. Antimicrobial Agents and Chemotherapy, 52(6), 2120-2129. https://doi.org/10.1128/AAC.01424-07.
Alghrair, Z. K., Fernig, D. G., & Ebrahimi, B. (2019). Enhanced Inhibition of Influenza Virus Infection by Peptide-Noble-Metal Nanoparticle Conjugates. Beilstein Journal of Nanotechnology, 10, 1038-1047. https://doi.org/10.3762/bjnano.10.104.
Andaloussi, S. E., Lehto, T., Mäger, I., Rosenthal-Aizman, K., Oprea, I. I., Simonson, O. E., Sork, H., Ezzat, K., Copolovici, D. M., Kurrikoff, K., Viola, J. R., Zaghloul, E. M., Sillard, R., Johansson, H. J., Said Hassane, F., Guterstam, P., Suhorutšenko, J., Moreno, P. M., Oskolkov, N., Langel, U. (2011). Design of a Peptide-Based Vector, PepFect6, for Efficient Delivery of siRNA in Cell Culture and Systemically in Vivo. Nucleic Acids Research, 39(9), 3972-3987. https://doi.org/10.1093/nar/gkq1299.
Battles, M. B., & McLellan, J. S. (2019). Respiratory Syncytial Virus Entry and How to Block it. Nature Reviews. Microbiology, 17(4), 233-245. https://doi.org/10.1038/s41579-019-0149-x.
Battles, M. B., Langedijk, J. P., Furmanova-Hollenstein, P., Chaiwatpongsakorn, S., Costello, H. M., Kwanten, L., Vranckx, L., Vink, P., Jaensch, S., Jonckers, T. H., Koul, A., Arnoult, E., Peeples, M. E., Roymans, D., & McLellan, J. S. (2016). Molecular Mechanism of Respiratory Syncytial Virus Fusion Inhibitors. Nature Chemical Biology, 12(2), 87-93. https://doi.org/10.1038/nchembio.1982.
Bawage, S. S., Tiwari, P. M., Singh, A., Dixit, S., Pillai, S. R., Dennis, V. A., & Singh, S. R. (2016). Gold Nanorods Inhibit Respiratory Syncytial Virus by Stimulating the Innate Immune Response. Nanomedicine : Nanotechnology, Biology, and Medicine, 12(8), 2299-2310. https://doi.org/10.1016/j.nano.2016.06.006.
Beauchemin, C. A. A., Kim, Y. I., Yu, Q., Ciaramella, G., & DeVincenzo, J. P. (2019). Uncovering Critical Properties of the Human Respiratory Syncytial Virus by Combining in Vitro Assays and in Silico Analyses. PLOS ONE, 14(4), e0214708. https://doi.org/10.1371/journal.pone.0214708.
Bechara, C., & Sagan, S. (2013). Cell-Penetrating Peptides : 20 Years Later, Where do We Stand ? FEBS Letters, 587(12), 1693-1702. https://doi.org/10.1016/j.febslet.2013.04.031.
Bellet-Amalric, E., Blaudez, D., Desbat, B., Graner, F., Gauthier, F., & Renault, A. (2000). Interaction of the Third Helix of Antennapedia Homeodomain and a Phospholipid Monolayer, Studied by Ellipsometry and PM-IRRAS at the Air-Water Interface. Biochimica et Biophysica Acta, 1467(1), 131-143. https://doi.org/10.1016/s0005-2736(00)00218-2.
Borchers, A. T., Chang, C., Gershwin, M. E., & Gershwin, L. J. (2013). Respiratory Syncytial Virus-A Comprehensive Review. Clinical Reviews in Allergy and Immunology, 45(3), 331-379. https://doi.org/10.1007/s12016-013-8368-9.
Brinkmann, V., Reichard, U., Goosmann, C., Fauler, B., Uhlemann, Y., Weiss, D. S., Weinrauch, Y., & Zychlinsky, A. (2004). Neutrophil Extracellular Traps Kill Bacteria. Science, 303(5663), 1532-1535. https://doi.org/10.1126/science.1092385.
Bultmann, H., & Brandt, C. R. (2002). Peptides Containing Membrane-Transiting Motifs Inhibit Virus Entry. Journal of Biological Chemistry, 277(39), 36018-36023. https://doi.org/10.1074/jbc.M204849200.
Bultmann, H., Teuton, J., & Brandt, C. R. (2007). Addition of a C-Terminal Cysteine Improves the Anti-Herpès Simplex Virus Activity of a Peptide Containing the Human Immunodeficiency Virus Type 1 TAT Protein Transduction Domain. Antimicrobial Agents and Chemotherapy, 51(5), 1596-1607. https://doi.org/10.1128/AAC.01009-06.
Carvajal, J. J., Avellaneda, A. M., Salazar-Ardiles, C., Maya, J. E., Kalergis, A. M., & Lay, M. K. (Hosts). (2019). Host Components Contributing to Respiratory Syncytial Virus Pathogenesis. Frontiers in Immunology, 10, 2152. https://doi.org/10.3389/fimmu.2019.02152.
Centers for Disease Control and Prevention (US). (2020). Respiratory Syncytial Virus Infections : Trends and Surveyllence, Retrieved 12/18/2020, 12/25 [Cited 2021 12/25/2021].
Chakravarty, M., & Vora, A. (2021). Nanotechnology-Based Antiviral therapeutics. Drug Delivery and Translational Research, 11(3), 748-787. https://doi.org/10.1007/s13346-020-00818-0.
Chu, X., Wu, B., Fan, H., Hou, J., Hao, J., Hu, J., Wang, B., Liu, G., Li, C., & Meng, S. (2016). PTD-Fused p53 as a Potential Antiviral Agent Directly Suppresses HBV Transcription and Expression. Antiviral Research, 127, 41-49. https://doi.org/10.1016/j.antiviral.2016.01.008.
Cochran, A. G. (2000). Antagonists of Protein-Protein Interactions. Chemistry and Biology, 7(4), R85-R94. https://doi.org/10.1016/s1074-5521(00)00106-x.
Collins, P. L., & Graham, B. S. (2008). Viral and Host Factors in Human Respiratory Syncytial Virus Pathogenesis. Journal of Virology, 82(5), 2040-2055. https://doi.org/10.1128/JVI.01625-07.
Collins, P. L., Fearns, R., & Graham, B. S. (2013). Respiratory Syncytial Virus : Virology, Reverse Genetics, and Pathogenesis of Disease. Current topics in Microbiology and Immunology, 372, 3-38. https://doi.org/10.1007/978-3-642-38919-1_1.
Collins, P. L., Hill, M. G., Cristina, J., & Grosfeld, H. (1996). Transcription Elongation Factor of Respiratory Syncytial Virus, A Nonsegmented Negative-Strand RNA Virus. Proceedings of the National Academy of Sciences of the United States of America, 93(1), 81-85. https://doi.org/10.1073/pnas.93.1.81.
Copolovici, D. M., Langel, K., Eriste, E., & Langel, Ü. (2014). Cell-Penetrating Peptides : Design, Synthesis, and Applications. ACS Nano, 8(3), 1972-1994. https://doi.org/10.1021/nn4057269.
Currie, S. M., Findlay, E. G., McHugh, B. J., Mackellar, A., Man, T., Macmillan, D., Wang, H., Fitch, P. M., Schwarze, J., & Davidson, D. J. (2013). The Human Cathelicidin LL-37 has Antiviral Activity Against Respiratory Syncytial Virus. PLOS ONE, 8(8), e73659. https://doi.org/10.1371/journal.pone.0073659.
Currie, S. M., Gwyer Findlay, E., McFarlane, A. J., Fitch, P. M., Böttcher, B., Colegrave, N., Paras, A., Jozwik, A., Chiu, C., Schwarze, J., & Davidson, D. J. (2016). Cathelicidins have Direct Antiviral Activity Against Respiratory Syncytial Virus in Vitro and Protective Function in Vivo in Mice and Humans. Journal of Immunology, 196(6), 2699-2710. https://doi.org/10.4049/jimmunol.1502478.
De Oliveira, E. C. L., Santana, K., Josino, L., Lima E Lima, A. H., & De Souza De Sales Júnior, C. (2021). Predicting Cell-Penetrating Peptides Using Machine Learning Algorithms and Navigating in their Chemical Space. Scientific Reports, 11(1), 7628. https://doi.org/10.1038/s41598-021-87134-w.
Delcroix, M., & Riley, L. W. (2010). Cell-Penetrating Peptides for Antiviral Drug Development. Pharmaceuticals, 3(3), 448-470. https://doi.org/10.3390/ph3030448.
Delshadi, R., Bahrami, A., McClements, D. J., Moore, M. D., & Williams, L. (2021). Development of Nanoparticle-Delivery Systems for Antiviral Agents : A Review. Journal of Controlled Release, 331, 30-44. https://doi.org/10.1016/j.jconrel.2021.01.017.
Derakhshankhah, H., & Jafari, S. (2018). Cell Penetrating Peptides : A Concise Review with Emphasis on Biomedical Applications. Biomedicine and Pharmacotherapy, 108, 1090-1096. https://doi.org/10.1016/j.biopha.2018.09.097.
Derossi, D., Chassaing, G., & Prochiantz, A. (1998). Trojan Peptides : the Penetratin System for Intracellular Delivery. Trends in Cell Biology, 8(2), 84-87. https://doi.org/10.1016/S0962-8924(98)80017-2.
Desale, K., Kuche, K., & Jain, S. (2021). Cell-Penetrating Peptides (CPPs): An Overview of Applications for Improving the Potential of Nanotherapeutics. Biomaterials Science, 9(4), 1153-1188. https://doi.org/10.1039/d0bm01755h.
Didierlaurent, A., Goulding, J., Patel, S., Snelgrove, R., Low, L., Bebien, M., Lawrence, T., van Rijt, L. S., Lambrecht, B. N., Sirard, J. C., & Hussell, T. (2008). Sustained Desensitization to Bacterial toll-Like Receptor Ligands After Resolution of Respiratory Influenza Infection. Journal of Experimental Medicine, 205(2), 323-329. https://doi.org/10.1084/jem.20070891.
Duchardt, F., Ruttekolk, I. R., Verdurmen, W. P. R., Lortat-Jacob, H., Bürck, J., Hufnagel, H., Fischer, R., van den Heuvel, M., Löwik, D. W. P. M., Vuister, G. W., Ulrich, A., de Waard, M., & Brock, R. (2009). A Cell-Penetrating Peptide Derived from Human Lactoferrin with Conformation-Dependent Uptake Efficiency. Journal of Biological Chemistry, 284(52), 36099-36108. https://doi.org/10.1074/jbc.M109.036426.
Dudas, R. A., & Karron, R. A. (1998). Respiratory Syncytial Virus Vaccines. Clinical Microbiology Reviews, 11(3), 430-439. https://doi.org/10.1128/CMR.11.3.430.
Elmquist, A., Hansen, M., & Langel, U. (2006). Structure-Activity Relationship Study of the Cell-Penetrating Peptide pVEC. Biochimica et Biophysica Acta, 1758(6), 721-729. https://doi.org/10.1016/j.bbamem.2006.05.013.
Fjell, C. D., Hiss, J. A., Hancock, R. E., & Schneider, G. (2011). Designing Antimicrobial Peptides : Form Follows Function. Nature Reviews. Drug Discovery, 11(1), 37-51. https://doi.org/10.1038/nrd3591.
Gao, C., Mao, S., Ditzel, H. J., Farnaes, L., Wirsching, P., Lerner, R. A., & Janda, K. D. (2002). A Cell-Penetrating Peptide from a Novel pVII-pIX Phage-Displayed Random Peptide Library. Bioorganic and Medicinal Chemistry, 10(12), 4057-4065. https://doi.org/10.1016/s0968-0896(02)00340-1.
Gautam, A., Chaudhary, K., Kumar, R., Sharma, A., Kapoor, P., Tyagi, A., Open Source Drug Discovery Consortium, & Raghava, G. P. (2013). In Silico Approaches for Designing Highly Effective Cell Penetrating Peptides. Journal of Translational Medicine, 11, 74. https://doi.org/10.1186/1479-5876-11-74.
Glezen, W. P., Paredes, A., Allison, J. E., Taber, L. H., & Frank, A. L. (1981). Risk of Respiratory Syncytial Virus Infection for Infants from Low-Income Families in Relationship to Age, Sex, Ethnic Group, and Maternal Antibody Level. Journal of Pediatrics, 98(5), 708-715. https://doi.org/10.1016/s0022-3476(81)80829-3.
González, P. A., Bueno, S. M., Carreño, L. J., Riedel, C. A., & Kalergis, A. M. (2012). Respiratory Syncytial Virus Infection and Immunity. Reviews in Medical Virology, 22(4), 230-244. https://doi.org/10.1002/rmv.1704.
Gorman, J. J., McKimm-Breschkin, J. L., Norton, R. S., & Barnham, K. J. (2001). Antiviral Activity and Structural Characteristics of the Nonglycosylated Central Subdomain of Human Respiratory Syncytial Virus Attachment (G) Glycoprotein. Journal of Biological Chemistry, 276(42), 38988-38994. https://doi.org/10.1074/jbc.M106288200.
Greulich, C., Diendorf, J., Simon, T., Eggeler, G., Epple, M., & Köller, M. (2011). Uptake and Intracellular Distribution of Silver Nanoparticles in Human Mesenchymal Stem Cells. Acta Biomaterialia, 7(1), 347-354. https://doi.org/10.1016/j.actbio.2010.08.003.
Gwyer Findlay, E., Currie, S. M., & Davidson, D. J. (2013). Cationic Host Defence Peptides : Potential as Antiviral therapeutics. BioDrugs, 27(5), 479-493. https://doi.org/10.1007/s40259-013-0039-0.
Hall, C. B., Kopelman, A. E., Douglas, R. G., Geiman, J. M., & Meagher, M. P. (1979). Neonatal Respiratory Syncytial Virus Infection. New England Journal of Medicine, 300(8), 393-396. https://doi.org/10.1056/NEJM197902223000803.
Higgins, D., Trujillo, C., & Keech, C. (2016). Advances in RSV Vaccine Research and Development - A Global Agenda. Vaccine, 34(26), 2870-2875. https://doi.org/10.1016/j.vaccine.2016.03.109.
Janai, H. K., Marks, M. I., Zaleska, M., & Stutman, H. R. (1990). Ribavirin : Adverse Drug Reactions, 1986 to 1988. Pediatric Infectious Disease Journal, 9(3), 209-211. https://doi.org/10.1097/00006454-199003000-00013.
Jartti, T., & Gern, J. E. (2017). Role of Viral Infections in the Development and Exacerbation of Asthma in Children. Journal of Allergy and Clinical Immunology, 140(4), 895-906. https://doi.org/10.1016/j.jaci.2017.08.003.
Jiang, T., Zhang, Z., Zhang, Y., Lv, H., Zhou, J., Li, C., Hou, L., & Zhang, Q. (2012). Dual-Functional Liposomes Based on pH-Responsive Cell-Penetrating Peptide and Hyaluronic Acid for Tumor-Targeted Anticancer Drug Delivery. Biomaterials, 33(36), 9246-9258. https://doi.org/10.1016/j.biomaterials.2012.09.027.
Jiao, C. Y., Delaroche, D., Burlina, F., Alves, I. D., Chassaing, G., & Sagan, S. (2009). Translocation and Endocytosis for Cell-Penetrating Peptide Internalization. Journal of Biological Chemistry, 284(49), 33957-33965. https://doi.org/10.1074/jbc.M109.056309.
Joshi, S., Bawage, S., Tiwari, P., Kirby, D., Perrie, Y., Dennis, V., & Singh, S. R. (2019). Liposomes : A Promising Carrier for Respiratory Syncytial Virus therapeutics. Expert Opinion on Drug Delivery, 16(9), 969-980. https://doi.org/10.1080/17425247.2019.1652268.
Kabanov, A. V., Lemieux, P., Vinogradov, S., & Alakhov, V. (2002). Pluronic Block Copolymers : Novel Functional Molecules for Gene Therapy. Advanced Drug Delivery Reviews, 54(2), 223-233. https://doi.org/10.1016/s0169-409x(02)00018-2.
Kamphuis, T., Stegmann, T., Meijerhof, T., Wilschut, J., & de Haan, A. (2013). A Virosomal Respiratory Syncytial Virus Vaccine Adjuvanted with Monophosphoryl Lipid a Provides Protection Against Viral Challenge without Priming for Enhanced Disease in Cotton Rats. Influenza and Other Respiratory Viruses, 7(6), 1227-1236. https://doi.org/10.1111/irv.12112.
Keogan, S., Passic, S., & Krebs, F. C. (2012). Infection by CXCR4-Tropic Human Immunodeficiency Virus Type 1 is Inhibited by the Cationic Cell-Penetrating Peptide Derived from HIV-1 Tat. International Journal of Peptides, 2012, 349427. https://doi.org/10.1155/2012/349427.
Kolli, D., Bao, X., & Casola, A. (2012). Human Metapneumovirus Antagonism of Innate Immune Responses. Viruses, 4(12), 3551-3571. https://doi.org/10.3390/v4123551.
Kolokoltsov, A. A., Deniger, D., Fleming, E. H., Roberts, N. J., Karpilow, J. M., & Davey, R. A. (2007). Small Interfering RNA Profiling Reveals Key Role of Clathrin-Mediated Endocytosis and Early Endosome Formation for Infection by Respiratory Syncytial Virus. Journal of Virology, 81(14), 7786-7800. https://doi.org/10.1128/JVI.02780-06.
Kota, S., Sabbah, A., Chang, T. H., Harnack, R., Xiang, Y., Meng, X., & Bose, S. (2008). Role of Human Beta-Defensin-2 During Tumor Necrosis Factor-Alpha/NF-kappaB-Mediated Innate Antiviral Response Against Human Respiratory Syncytial Virus. Journal of Biological Chemistry, 283(33), 22417-22429. https://doi.org/10.1074/jbc.M710415200.
Krajewski, K., Marchand, C., Long, Y. Q., Pommier, Y., & Roller, P. P. (2004). Synthesis and HIV-1 Integrase Inhibitory Activity of Dimeric and Tetrameric Analogs of Indolicidin. Bio Organic and Medicinal Chemistry Letters, 14(22), 5595-5598. https://doi.org/10.1016/j.bmcl.2004.08.061.
Kurrikoff, K., Gestin, M., & Langel, Ü. (2016). Recent in Vivo Advances in Cell-Penetrating Peptide-Assisted Drug Delivery. Expert Opinion on Drug Delivery, 13(3), 373-387. https://doi.org/10.1517/17425247.2016.1125879.
Lalonde, M. S., Lobritz, M. A., Ratcliff, A., Chamanian, M., Athanassiou, Z., Tyagi, M., Wong, J., Robinson, J. A., Karn, J., Varani, G., & Arts, E. J. (2011). Inhibition of Both HIV-1 Reverse Transcription and Gene Expression by a Cyclic Peptide that Binds the Tat-Transactivating Response Element (TAR) RNA. PLOS Pathogens, 7(5). https://doi.org/10.1371/journal.ppat.1002038.
Lambert, D. M., Barney, S., Lambert, A. L., Guthrie, K., Medinas, R., Davis, D. E., Bucy, T., Erickson, J., Merutka, G., & Petteway, S. R. (1996). Peptides from Conserved Regions of Paramyxovirus Fusion (F) Proteins are Potent Inhibitors of Viral Fusion. Proceedings of the National Academy of Sciences of the United States of America, 93(5), 2186-2191. https://doi.org/10.1073/pnas.93.5.2186.
Lara, H. H., Ayala-Nuñez, N. V., Ixtepan-Turrent, L., & Rodriguez-Padilla, C. (2010). Mode of Antiviral Action of Silver Nanoparticles Against HIV-1. Journal of Nanobiotechnology, 8, 1. https://doi.org/10.1186/1477-3155-8-1.
Madani, F., Lindberg, S., Langel, U., Futaki, S., & Gräslund, A. (2011). Mechanisms of Cellular Uptake of Cell-Penetrating Peptides. Journal of Biophysics, 2011, 414729. https://doi.org/10.1155/2011/414729.
Maeda, H. (2010). Tumor-Selective Delivery of Macromolecular Drugs Via the EPR Effect : Background and Future Prospects. Bioconjugate Chemistry, 21(5), 797-802. https://doi.org/10.1021/bc100070g.
Mann, D. A., & Frankel, A. D. (1991). Endocytosis and Targeting of Exogenous HIV-1 Tat Protein. EMBO Journal, 10(7), 1733-1739. https://doi.org/10.1002/j.1460-2075.1991.tb07697.x.
Mayor, S., & Pagano, R. E. (2007). Pathways of Clathrin-Independent Endocytosis. Nature Reviews. Molecular Cell Biology, 8(8), 603-612. https://doi.org/10.1038/nrm2216.
McErlean, E. M., Ziminska, M., McCrudden, C. M., McBride, J. W., Loughran, S. P., Cole, G., Mulholland, E. J., Kett, V., Buckley, N. E., Robson, T., Dunne, N. J., & McCarthy, H. O. (2021). Rational Design and Characterisation of a Linear Cell Penetrating Peptide for Non-Viral Gene Delivery. Journal of Controlled Release, 330, 1288-1299. https://doi.org/10.1016/j.jconrel.2020.11.037.
Milletti, F. (2012). Cell-Penetrating Peptides : Classes, Origin, and Current Landscape. Drug Discovery today, 17(15-16), 850-860. https://doi.org/10.1016/j.drudis.2012.03.002.
Mino, T., Mori, T., Aoyama, Y., & Sera, T. (2008). Cell-Permeable Artificial Zinc-Finger Proteins as Potent Antiviral Drugs for Human Papillomaviruses. Archives of Virology, 153(7), 1291-1298. https://doi.org/10.1007/s00705-008-0125-7.
Mishra, A., Lai, G. H., Schmidt, N. W., Sun, V. Z., Rodriguez, A. R., tong, R., Tang, L., Cheng, J., Deming, T. J., Kamei, D. T., & Wong, G. C. (2011). Translocation of HIV TAT Peptide and Analogues Induced by Multiplexed Membrane and Cytoskeletal Interactions. Proceedings of the National Academy of Sciences of the United States of America, 108(41), 16883-16888. https://doi.org/10.1073/pnas.1108795108.
Moghadamtousi, S. Z., Kadir, H. A., Hassandarvish, P., Tajik, H., Abubakar, S., & Zandi, K. (2014). A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. BioMed Research International. https://doi.org/10.1155/2014/186864.
Morris, D., Ansar, M., Speshock, J., Ivanciuc, T., Qu, Y., Casola, A., & Garofalo, R. (2019). Antiviral and Immunomodulatory Activity of Silver Nanoparticles in Experimental RSV Infection. Viruses, 11(8). https://doi.org/10.3390/v11080732.
Munyendo, W. L., Lv, H., Benza-Ingoula, H., Baraza, L. D., & Zhou, J. (2012). Cell Penetrating Peptides in the Delivery of Biopharmaceuticals. Biomolecules, 2(2), 187-202. https://doi.org/10.3390/biom2020187.
Nascimento-Carvalho, A. C., Ruuskanen, O., & Nascimento-Carvalho, C. M. (2016). Comparison of the Frequency of Bacterial and Viral Infections Among Children with Community-Acquired Pneumonia Hospitalized Across Distinct Severity Categories: A Prospective Cross-Sectional Study. BMC Pediatrics, 16, 105. https://doi.org/10.1186/s12887-016-0645-3.
Nestor, J. J. (2009). The Medicinal Chemistry of Peptides. Current Medicinal Chemistry, 16(33), 4399-4418. https://doi.org/10.2174/092986709789712907.
Oehlke, J., Krause, E., Wiesner, B., Beyermann, M., & Bienert, M. (1997). Extensive Cellular Uptake into Endothelial Cells of an Amphipathic Beta-Sheet Forming Peptide. FEBS Letters, 415(2), 196-199. https://doi.org/10.1016/s0014-5793(97)01123-x.
Oehlke, J., Scheller, A., Wiesner, B., Krause, E., Beyermann, M., Klauschenz, E., Melzig, M., & Bienert, M. (1998). Cellular Uptake of an Alpha-Helical Amphipathic Model Peptide with the Potential to Deliver Polar Compounds into the Cell Interior Non-Endocytically. Biochimica et Biophysica Acta, 1414(1-2), 127-139. https://doi.org/10.1016/s0005-2736(98)00161-8.
Ogra, P. L. (2004). Respiratory Syncytial Virus : the Virus, the Disease and the Immune Response. Paediatric Respiratory Reviews, 5, Suppl. A, S119-S126. https://doi.org/10.1016/s1526-0542(04)90023-1.
Okiro, E. A., Ngama, M., Bett, A., Cane, P. A., Medley, G. F., & James Nokes, D. (2008). Factors Associated with Increased Risk of Progression to Respiratory Syncytial Virus-Associated Pneumonia in Young Kenyan Children. Tropical Medicine and International Health, 13(7), 914-926. https://doi.org/10.1111/j.1365-3156.2008.02092.x.
Otsuka, H., Nagasaki, Y., & Kataoka, K. (2003). Pegylated Nanoparticles for Biological and Pharmaceutical Applications. Advanced Drug Delivery Reviews, 55(3), 403-419. https://doi.org/10.1016/s0169-409x(02)00226-0.
Padari, K., Koppel, K., Lorents, A., Hällbrink, M., Mano, M., Pedroso de Lima, M. C., & Pooga, M. (2010). S4(13) -PV Cell-Penetrating Peptide forms Nanoparticle-Like Structures to Gain Entry into Cells. Bioconjugate Chemistry, 21(4), 774-783. https://doi.org/10.1021/bc900577e.
Patra, J. K., Das, G., Fraceto, L. F., Campos, E. V. R., Rodriguez-Torres, M. D. P., Acosta-Torres, L. S., Diaz-Torres, L. A., Grillo, R., Swamy, M. K., Sharma, S., Habtemariam, S., & Shin, H. S. (2018). Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. Journal of Nanobiotechnology, 16(1), 71. https://doi.org/10.1186/s12951-018-0392-8.
Perk, Y., & Özdil, M. (2018). Respiratory Syncytial Virüs Infections in Neonates and Infants. Turk Pediatri Arsivi, 53(2), 63-70. https://doi.org/10.5152/TurkPediatriArs.2018.6939.
Persson, B. D., Jaffe, A. B., Fearns, R., & Danahay, H. (2014). Respiratory Syncytial Virus Can Infect Basal Cells and Alter Human Airway Epithelial Differentiation. PLOS ONE, 9(7). https://doi.org/10.1371/journal.pone.0102368.
Pickles, R. J. (2013). Human Airway Epithelial Cell Cultures for Modeling Respiratory Syncytial Virus Infection. Current topics in Microbiology and Immunology, 372, 371-387. https://doi.org/10.1007/978-3-642-38919-1_19.
Pickles, R. J., & DeVincenzo, J. P. (2015). Respiratory Syncytial Virus (RSV) and its Propensity for Causing Bronchiolitis. Journal of Pathology, 235(2), 266-276. https://doi.org/10.1002/path.4462.
Pooga, M., Hällbrink, M., Zorko, M., & Langel, U. (1998). Cell Penetration by Transportan. FASEB Journal, 12(1), 67-77. https://doi.org/10.1096/fasebj.12.1.67.
Pouny, Y., Rapaport, D., Mor, A., Nicolas, P., & Shai, Y. (1992). Interaction of Antimicrobial Dermaseptin and its Fluorescently Labeled Analogues with Phospholipid Membranes. Biochemistry, 31(49), 12416-12423. https://doi.org/10.1021/bi00164a017.
Powell, K. (2021). The Race to Make Vaccines for a Dangerous Respiratory Virus. Nature, 600(7889), 379-380. https://doi.org/10.1038/d41586-021-03704-y.
Qiao, J., Li, A., & Jin, X. (2011). TSLP from RSV-Stimulated Rat Airway Epithelial Cells Activates Myeloid Dendritic Cells. Immunology and Cell Biology, 89(2), 231-238. https://doi.org/10.1038/icb.2010.85.
Ramos-Fernández, J. M., Moreno-Pérez, D., Gutiérrez-Bedmar, M., Hernández-Yuste, A., Cordón-Martínez, A. M., Milano-Manso, G., & Urda-Cardona, A. (2017). Predicción de la Evolución de la Bronquiolitis Por Virus Respiratorio Sincitial en Lactantes Menores de 6 Meses [Prediction of Severe Course in Infants with RSV Bronchiolitis under 6 Months. Spain]. Revista Espanola de Salud Publica, 91.
Reissmann, S. (2014). Cell Penetration : Scope and Limitations by the Application of Cell-Penetrating Peptides. Journal of Peptide Science, 20(10), 760-784. https://doi.org/10.1002/psc.2672.
Renukuntla, J., Vadlapudi, A. D., Patel, A., Boddu, S. H., & Mitra, A. K. (2013). Approaches for Enhancing Oral Bioavailability of Peptides and Proteins. International Journal of Pharmaceutics, 447(1-2), 75-93. https://doi.org/10.1016/j.ijpharm.2013.02.030.
Rijsbergen, L. C., van Dijk, L. L. A., Engel, M. F. M., de Vries, R. D., & de Swart, R. L. (2021). In Vitro Modelling of Respiratory Virus Infections in Human Airway Epithelial Cells - A Systematic Review. Frontiers in Immunology, 12, 683002. https://doi.org/10.3389/fimmu.2021.683002.
Ruseska, I., & Zimmer, A. (2020). Internalization Mechanisms of Cell-Penetrating Peptides. Beilstein Journal of Nanotechnology, 11, 101-123. https://doi.org/10.3762/bjnano.11.10.
Russell, C. D., Unger, S. A., Walton, M., & Schwarze, J. (2017). The Human Immune Response to Respiratory Syncytial Virus Infection. Clinical Microbiology Reviews, 30(2), 481-502. https://doi.org/10.1128/CMR.00090-16.
Russi, J. C., Delfraro, A., Borthagaray, M. D., Velazquez, B., García-Barreno, B., & Hortal, M. (1993). Evaluation of Immunoglobulin E-Specific Antibodies and Viral Antigens in Nasopharyngeal Secretions of Children with Respiratory Syncytial Virus Infections. Journal of Clinical Microbiology, 31(4), 819-823. https://doi.org/10.1128/jcm.31.4.819-823.1993.
Ruuskanen, O., & Ogra, P. L. (1993). Respiratory Syncytial Virus. Current Problems in Pediatrics, 23(2), 50-79. https://doi.org/10.1016/0045-9380(93)90003-u.
Sadeghian, I., Heidari, R., Sadeghian, S., Raee, M. J., & Negahdaripour, M. (2022). Potential of Cell-Penetrating Peptides (Cpps) in Delivery of Antiviral therapeutics and Vaccines. European Journal of Pharmaceutical Sciences, 169, 106094. https://doi.org/10.1016/j.ejps.2021.106094.
Scott, J. A., Wonodi, C., Moïsi, J. C., Deloria-Knoll, M., DeLuca, A. N., Karron, R. A., Bhat, N., Murdoch, D. R., Crawley, J., Levine, O. S., O'Brien, K. L., Feikin, D. R., & Pneumonia Methods Working Group. (2012). The Definition of Pneumonia, the Assessment of Severity, and Clinical Standardization in the Pneumonia Etiology Research for Child Health Study. Clinical Infectious Diseases, 54, (Suppl 2), S109-S116. https://doi.org/10.1093/cid/cir1065.
Shepherd, N. E., Hoang, H. N., Desai, V. S., Letouze, E., Young, P. R., & Fairlie, D. P. (2006). Modular Alpha-Helical Mimetics with Antiviral Activity Against Respiratory Syncitial Virus. Journal of the American Chemical Society, 128(40), 13284-13289. https://doi.org/10.1021/ja064058a.
Shi, T., McAllister, D. A., O'Brien, K. L., Simoes, E. A. F., Madhi, S. A., Gessner, B. D., Polack, F. P., Balsells, E., Acacio, S., Aguayo, C., Alassani, I., Ali, A., Antonio, M., Awasthi, S., Awori, J. O., Azziz-Baumgartner, E., Baggett, H. C., Baillie, V. L., Balmaseda, A., RSV Global Epidemiology Network. (2017). Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children in 2015 : A Systematic Review and Modelling Study. Lancet, 390(10098), 946-958. https://doi.org/10.1016/S0140-6736(17)30938-8.
Shilovskiy, I. P., andreev, S. M., Kozhikhova, K. V., Nikolskii, A. A., & Khaitov, M. R. (2019). Prospects for the Use of Peptides Against Respiratory Syncytial Virus. Molekuliarnaia Biologiia, 53(4), 541-560. https://doi.org/10.1134/S002689841904013X.
Silhol, M., Tyagi, M., Giacca, M., Lebleu, B., & Vivès, E. (2002). Different Mechanisms for Cellular Internalization of the HIV-1 Tat-Derived Cell Penetrating Peptide and Recombinant Proteins Fused to Tat Tat. European Journal of Biochemistry, 269(2), 494-501. https://doi.org/10.1046/j.0014-2956.2001.02671.x.
Silva, S., Almeida, A. J., & Vale, N. (2019). Combination of Cell-Penetrating Peptides with Nanoparticles for Therapeutic Application : A Review. Biomolecules, 9(1). https://doi.org/10.3390/biom9010022.
Simões, E. A. F., Bont, L., Manzoni, P., Fauroux, B., Paes, B., Figueras-Aloy, J., Checchia, P. A., & Carbonell-Estrany, X. (2018). Past, Present and Future Approaches to the Prevention and Treatment of Respiratory Syncytial Virus Infection in Children. Infectious Diseases and therapy, 7(1), 87-120. https://doi.org/10.1007/s40121-018-0188-z.
Singh, S. R. (2012). Anti Respiratory Syncytial Virus Peptide Functionalized Gold Nanoparticles, A.S. University, U.S.
Soto, J. A., Gálvez, N. M. S., Pacheco, G. A., Bueno, S. M., & Kalergis, A. M. (2020). Antibody Development for Preventing the Human Respiratory Syncytial Virus Pathology. Molecular Medicine, 26(1), 35. https://doi.org/10.1186/s10020-020-00162-6.
Srinivasakumar, N., Ogra, P. L., & Flanagan, T. D. (1991). Characteristics of Fusion of Respiratory Syncytial Virus With HEp-2 Cells as Measured by R18 Fluorescence Dequenching Assay. Journal of Virology, 65(8), 4063-4069. https://doi.org/10.1128/JVI.65.8.4063-4069.1991.
Sun, Z., Pan, Y., Jiang, S., & Lu, L. (2013). Respiratory Syncytial Virus Entry Inhibitors Targeting the F Protein. Viruses, 5(1), 211-225. https://doi.org/10.3390/v5010211.
Tang, Z., Zhang, X., Shu, Y., Guo, M., Zhang, H., & Tao, W. (2021). Insights from Nanotechnology in COVID-19 Treatment. Nano today, 36. https://doi.org/10.1016/j.nantod.2020.101019.
Taylor, G. (2017). Animal Models of Respiratory Syncytial Virus Infection. Vaccine, 35(3), 469-480. https://doi.org/10.1016/j.vaccine.2016.11.054.
Telcian, A. G., Laza-Stanca, V., Edwards, M. R., Harker, J. A., Wang, H., Bartlett, N. W., Mallia, P., Zdrenghea, M. T., Kebadze, T., Coyle, A. J., Openshaw, P. J., Stanciu, L. A., & Johnston, S. L. (2011). RSV-Induced Bronchial Epithelial Cell PD-L1 Expression Inhibits CD8+ T Cell Nonspecific Antiviral Activity. Journal of Infectious Diseases, 203(1), 85-94. https://doi.org/10.1093/infdis/jiq020.
Teng, M. N., & Collins, P. L. (1998). Identification of the Respiratory Syncytial Virus Proteins Required for Formation and Passage of Helper-Dependent Infectious Particles. Journal of Virology, 72(7), 5707-5716. https://doi.org/10.1128/JVI.72.7.5707-5716.1998.
Ter-Avetisyan, G., Tünnemann, G., Nowak, D., Nitschke, M., Herrmann, A., Drab, M., & Cardoso, M. C. (2009). Cell Entry of Arginine-Rich Peptides is Independent of Endocytosis. Journal of Biological Chemistry, 284(6), 3370-3378. https://doi.org/10.1074/jbc.M805550200.
Thorén, P. E., Persson, D., Isakson, P., Goksör, M., Onfelt, A., & Nordén, B. (2003). Uptake of Analogs of Penetration, Tat. Biochemical and Biophysical Research Communications, 307(1), 100-107. https://doi.org/10.1016/s0006-291x(03)01135-5.
Tiwari, P. M., Eroglu, E., Bawage, S. S., Vig, K., Miller, M. E., Pillai, S., Dennis, V. A., & Singh, S. R. (2014). Enhanced Intracellular Translocation and Biodistribution of Gold Nanoparticles Functionalized with a Cell-Penetrating Peptide (VG-21) from Vesicular Stomatitis Virus. Biomaterials, 35(35), 9484-9494. https://doi.org/10.1016/j.biomaterials.2014.07.032.
Tünnemann, G., Ter-Avetisyan, G., Martin, R. M., Stöckl, M., Herrmann, A., & Cardoso, M. C. (2008). Live-Cell Analysis of Cell Penetration Ability and toxicity of Oligo-Arginines. Journal of Peptide Science, 14(4), 469-476. https://doi.org/10.1002/psc.968.
Utley, T. J., Ducharme, N. A., Varthakavi, V., Shepherd, B. E., Santangelo, P. J., Lindquist, M. E., Goldenring, J. R., & Crowe, J. E. (2008). Respiratory Syncytial Virus Uses a Vps4-Independent Budding Mechanism Controlled by Rab11-FIP2. Proceedings of the National Academy of Sciences of the United States of America, 105(29), 10209-10214. https://doi.org/10.1073/pnas.0712144105.
Vanheule, V., Vervaeke, P., Mortier, A., Noppen, S., Gouwy, M., Snoeck, R., andrei, G., Van Damme, J., Liekens, S., & Proost, P. (2016). Basic Chemokine-Derived Glycosaminoglycan Binding Peptides Exert Antiviral Properties Against Dengue Virus Serotype 2, Herpès Simplex Virus-1 and Respiratory Syncytial Virus. Biochemical Pharmacology, 100, 73-85. https://doi.org/10.1016/j.bcp.2015.11.001.
Verdurmen, W. P., Thanos, M., Ruttekolk, I. R., Gulbins, E., & Brock, R. (2010). Cationic Cell-Penetrating Peptides Induce Ceramide Formation Via Acid Sphingomyelinase : Implications for Uptake. Journal of Controlled Release, 147(2), 171-179. https://doi.org/10.1016/j.jconrel.2010.06.030.
Veronese, F. M., & Mero, A. (2008). The Impact of Pegylation on Biological Therapies. BioDrugs, 22(5), 315-329. https://doi.org/10.2165/00063030-200822050-00004.
Wadia, J. S., Stan, R. V., & Dowdy, S. F. (2004). Transducible TAT-HA Fusogenic Peptide Enhances Escape of TAT-Fusion Proteins After Lipid Raft Macropinocytosis. Nature Medicine, 10(3), 310-315. https://doi.org/10.1038/nm996.
Wang, D., Cummins, C., Bayliss, S., Sandercock, J., & Burls, A. (2008). Immunoprophylaxis Against Respiratory Syncytial Virus (RSV) With Palivizumab in Children : A Systematic Review and Economic Evaluation. Health Technology Assessment, 12(36), iii, ix-x, 1-86. https://doi.org/10.3310/hta12360.
Wang, E., Sun, X., Qian, Y., Zhao, L., Tien, P., & Gao, G. F. (2003). Both Heptad Repeats of Human Respiratory Syncytial Virus Fusion Protein are Potent Inhibitors of Viral Fusion. Biochemical and Biophysical Research Communications, 302(3), 469-475. https://doi.org/10.1016/s0006-291x(03)00197-9.
Wang, F., Wang, Y., Zhang, X., Zhang, W., Guo, S., & Jin, F. (2014). Recent Progress of Cell-Penetrating Peptides as New Carriers for Intracellular Cargo Delivery. Journal of Controlled Release, 174, 126-136. https://doi.org/10.1016/j.jconrel.2013.11.020.
Wang, Y. F., Xu, X., Fan, X., Zhang, C., Wei, Q., Wang, X., Guo, W., Xing, W., Yu, J., Yan, J. L., & Liang, H. P. (2011). A Cell-Penetrating Peptide Suppresses Inflammation by Inhibiting NF-κB Signaling. Molecular therapy, 19(10), 1849-1857. https://doi.org/10.1038/mt.2011.82.
Welliver, R. C. (2003). Respiratory Syncytial Virus and Other Respiratory Viruses. Pediatric Infectious Disease Journal, 22(2), Suppl., S6-S10, discussion S10-S12. https://doi.org/10.1097/01.inf.0000053880.92496.db.
Welliver, R. C. (2003). Review of Epidemiology and Clinical Risk Factors for Severe Respiratory Syncytial Virus (RSV) Infection. Journal of Pediatrics, 143(5), Suppl., S112-S117. https://doi.org/10.1067/s0022-3476(03)00508-0.
Xie, J., Bi, Y., Zhang, H., Dong, S., Teng, L., Lee, R. J., & Yang, Z. (2020). Cell-Penetrating Peptides in Diagnosis and Treatment of Human Diseases: From Preclinical Research to Clinical Application. Frontiers in Pharmacology, 11, 697. https://doi.org/10.3389/fphar.2020.00697.
Yadavalli, T., & Shukla, D. (2017). Role of Metal and Metal Oxide Nanoparticles as Diagnostic and Therapeutic Tools for Highly Prevalent Viral Infections. Nanomedicine : Nanotechnology, Biology, and Medicine, 13(1), 219-230. https://doi.org/10.1016/j.nano.2016.08.016.
Zhang, L., Peeples, M. E., Boucher, R. C., Collins, P. L., & Pickles, R. J. (2002). Respiratory Syncytial Virus Infection of Human Airway Epithelial Cells is Polarized, Specific to Ciliated Cells, and Without Obvious Cytopathology. Journal of Virology, 76(11), 5654-5666. https://doi.org/10.1128/jvi.76.11.5654-5666.2002.
Zhang, P., Moreno, R., Lambert, P. F., & DiMaio, D. (2020). Cell-Penetrating Peptide Inhibits Retromer-Mediated Human Papillomavirus Trafficking During Virus Entry. Proceedings of the National Academy of Sciences of the United States of America, 117(11), 6121-6128. https://doi.org/10.1073/pnas.1917748117.
Ziegler, A., Blatter, X. L., Seelig, A., & Seelig, J. (2003). Protein Transduction Domains of HIV-1 and SIV TAT Interact with Charged Lipid Vesicles. Binding Mechanism and Thermodynamic Analysis. Biochemistry, 42(30), 9185-9194. https://doi.org/10.1021/bi0346805.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2023. All Rights Reserved.