
A comparative study of LOW-DENSITY Polyethylene shopping carry bag degrading bacteria isolated from marine and garden soil
Ramya Sugandhi Katru
1![]()
![]() ,
Reshma Ladi 1
,
Reshma Ladi 1![]()
![]() ,
Husam Talib Hamzah 2
,
Husam Talib Hamzah 2![]()
![]() , Sridevi Velluru 3
, Sridevi Velluru 3![]()
![]() , B. K Babu 3
, B. K Babu 3
1 M.
Tech Biotechnology, Department of Chemical Engineering, Andhra University,
Vishakhapatnam-530003, India
2 Ph.D.
Scholar, Department of Chemical Engineering, Andhra University,
Vishakhapatnam-530003, India
3Professor, Department of Chemical Engineering, Andhra University,
Vishakhapatnam-530003, India
|
|
ABSTRACT |
||
|
An attempt was
made to investigate the extent of biodegradability of LDPE by
six strains were isolated from marine and garden soil. The phenotypic
fingerprint, similar to the Gen III biolog, was utilised to activate the
substrate utilisation of strains, which were identified as Paenibacillus
castanea and Riemerella anatipestifer. The optimal conditions for both was found to be pH of 7.1, temperature of 37oC, contact
time of 72hrs, LDPE weight & inoculums volume of Paenibacillus
castanea; 0.030 g & 3 % (v/v) for Riemerella anatipestifer; 0.042
g & 4% (v/v). LDPE degradation was confirmed by the weight loss which was
found to be 7.30 % for Paenibacillus castanea & 5.40 % for Riemerella
anatipestifer after an incubation of 35 days. The present study suggest that Paenibacillus castanea is efficient,
cost effective, eco friendly and safe approach for the elimination of LDPE
compared to Riemerella anatipestifer from the environment. The changes in the
functional group of Paenibacillus castanea was
further detected by FTIR. These results indicate that Paenibacillus castanea
can prove to be a suitable candidate for LDPE treatment without causing any
harm to our health or environment. |
|||
|
Received 12 May 2023 Accepted 13 June 2023 Published 30 June 2023 Corresponding Author Ramya
Sugandhi Katru, Ramyasugandhi111@gmail.com DOI 10.29121/granthaalayah.v11.i6.2023.5148 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2023 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Bio Degradation, Fourier Transform-Infrared Spectroscopy (FTIR), GEN III Biolog, LDPE, Paenibacillus Castanea & Riemerella Anatipestifer Graphical
Abstract
|
|||
1. INTRODUCTION
Due to its low production cost, the demand for plastic has been steadily rising over the past few decades Alshehrei (2017). To support the extensive usage of plastic products, global plastic manufacturing increased from 204 million tonnes to 348 million tonnes in 2017 Plastic Europe, (2018). Plastic waste's inability to degrade causes it to continually accumulate in the environment, posing a major hazard to practically all life forms Vatseldutt (2014). All sorts of terrestrial ecosystems, including deserts, forests, grasslands, and polar regions, are affected by the garbage in a significant way. 2020 (Kartikey Kumar Gupta and associates) Since plastics cannot or do not biodegrade well, they accumulate in the environment, causing extensive contamination and harming both marine and terrestrial species Raddadi and Fava (2019), Li et al. (2020). Plastics damage animals' digestive systems and enter the food chain, causing them to become ingested by other creatures and contributing to flooding by obstructing the water drainage system Muhonja et al. (2018). Long-term plastics buildup in soil even modifies the makeup of the microbial population there Huang et al. (2019), Fei et al. (2020). Despite the fact that incineration reduces the amount of plastic waste, it nevertheless produces secondary air pollutants such carbon monoxide and nitrogen oxides Ru et al. (2020). Microplastics (MP), which are produced by the weathering and degradation of plastic, travel towards rivers, ponds, lakes, oceans, and agricultural areas and have a negative impact on them Vatseldutt (2014). The most widely used commercial plastics are polyethylene (PE) (low density, or LDPE, and high density, or HDPE), polypropylene, polystyrene, polyvinyl chloride (PVC), polyamide (PA), and polyethylene terephthalate (PET). typically, not susceptible to biodegradation Danso et al. (2019)
Low-density polyethylene (LDPE) is a popular thermoplastic that is not biodegradable Okunola et al. (2019). Researchers are striving to transform non-biodegradable thermoplastics into biodegradable materials in order to address the environmental problem of non-biodegradable thermoplastics Das and Kumar (2015). Additionally, these synthetic polymers are typically not biodegradable until they are broken down into little, easily assimilated particles by microorganisms Francis et al. (2010), Sen and Raut (2015), Das and Kumar (2014).
Bacteria (Pseudomonas, Streptococcus, Staphylococcus, Micrococcus, Moraxella), fungi (Aspergillusniger, Aspergillusglaucus), Actinomycetes etc., and the Saccharomonospora genus were identified as the microbial species linked to the degrading polymers Gupta and Devi (2020).
Dey et al. (2020) investigated the ability of drilling fluids based on bentonite from deep subsurface drilling operations and aerobic bacteria enriched from municipal waste dumpsites to degrade LDPE. The pH changed to an acidic state, there was significant weight loss of the LDPE beads (8%), and there was biofilm cell growth surrounding the beads (CFU count 105-106/cm2) for two samples (P and DF2). The highly hydrophobic cell surfaces of the obtained concentrated microbial consortia (65–90%) validated their potential for LDPE adhesion and biofilm formation. From P and DF2 enrichments, pure cultures of two LDPE-degrading bacterial strains connected to Stenotrophomonas sp. and Achromobacter sp. were obtained. The 16S rRNA gene sequencing of these isolates demonstrated their taxonomic distinction. In this study, we used GEN-III to isolate and characterise the LDPE-degrading bacteria from the marine and garden soil samples in order to optimise the physiological parameters that affect the biodegradation of the obtained LDPE films and changes in the functional groups are detected by FTIR.
2. Materials and methods
2.1. Sample Collection
The bacterial species were isolated from the soil samples collected from RK beach and also from graden soil of Andhra University Campus premises, Visakhapatnam. The soil samples were collected from a depth of almost 5 cms in sterile containers and air dried in the laboratory at room temperature.
2.2. Pre-treatment of LDPE
Low density polyethylene (LDPE), which is used to make milk jugs and shopping bags, was obtained from Visakha Milk Dairy and a nearby market in Visakhapatnam. It was then cut into 1 x 1 cm discs and put into a sterile beaker with distilled water, where it was agitated for an hour. Additionally, they were aseptically exposed for 30 minutes to a 70% (v/v) ethanol solution. The LDPE discs were then put onto a clean petridish. The LDPE discs were air dried and weighted in fix mass before being finished.
2.3. Isolation and Screening of LDPE degrading microorganisms
Polyethylene degrading species were isolated from plastic adhered soil by clear zone method in our previous study and designated as S6 and A3. The cultures were maintained on nutrient agar and nutrient broth for further biodegradation studies.
2.4. GEN III
BIOLOG
The GEN III programme is used to determine the degree of ambiguity for genes and species, and it offers a clear indication for identification at the gene and species level. The unrivalled spectrum of gram negative and gram positive microorganisms can be used with the new GEN III redox chemistry. In addition to analysing and evaluating the cell's capacity to break down all key classes of biochemicals, GEN III also pinpoints other crucial physiological characteristics like pH, salt and reducing power, lactic acid tolerance, and chemical sensitivity. Isolated bacteria were recognised using the new GEN III Microplates TM test panel for the Biolog system. A "Phenotypic Fingerprint" of the microorganism used to identify them to a species level makes up the test panel. Using the GEN III Micro-PlatesTM, Gram-positive and Gram-negative microorganisms can be examined in the same test panel. 23 chemical sensitivity assays and 71 carbon sources make up the test panel.
A simple, straight
forward procedure:
The straightforward steps of isolation, inoculation, incubation, and reading are used to identify microorganisms in GEN III Biolog.
· Prepare an agar-based pure bacterial culture from an isolate.
· Inoculum should be prepared at the desired cell density.
· Overnight, inoculate the BiologMicroplate.
· Observe the reaction pattern while you incubate the plate. Enter the reaction pattern to get an ID result.
2.4.1. Microbial identification databases for biolog systems
The BiologMicrobial Identification System makes use of metabolic traits. It is predicated on the idea that a species of bacteria creates a distinct metabolic fingerprint based on a variety of carbon sources and biochemicals. The cultivated bacteria are examined for their ability to use various carbon sources and biochemicals that have been dried and pre-filled into a 96-well test plate. Utilising nutrients, cells breathe out and generate energy, which causes the patented Tetrazolium dye to break down and produce a distinctive purple colour. The distinct metabolic profile in Figure 1 is captured using Biolog data collecting software (Retrospect software), which is then compared to hundreds of other profiles (corresponding to thousands of species) saved in Biolog databases. The computer shows the species if the profile matches.
Figure 1
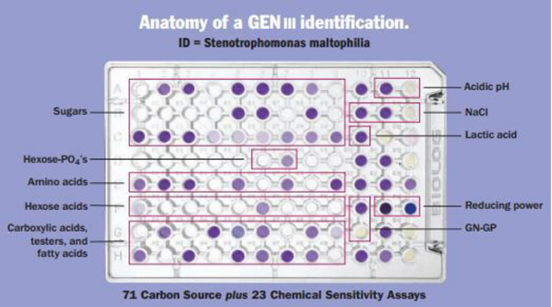
|
Figure 1 Anatomy of Gen III Identification |
2.5. Determination of the dry weight of the residual LDPE
According to Chatterjee et al. (2010), the dry weight of residual LDPE was calculated. The surface of the LDPE was treated with a 2% (v/v) aqueous sodium dodecyl sulphate (SDS) solution for four hours at 50 °C to remove the bacterial biofilm colonising it. The films were further cleaned with distilled water after the SDS wash. The cleaned LDPE films were assembled on filter paper and left to dry at 60 °C overnight. The weight loss of the films was calculated using the following formula, as per ASTM International (1996), after drying: WL (%) = [(mi mr)/mi] 100. Here, WL stands for weight loss of samples (%), mi for initial sample weight (g), and mr for retention weight of samples (g).
2.6. Determination of bacterial growth rate
According to the method, the growth of the bacterial strains in the liquid media due to the presence of LDPE film Chatterjee et al. (2010). Regular monitoring of the bacterial growth during the biodegradation investigation confirmed the use of LDPE as the exclusive carbon source. The optical density (660 nm) of the culture broth was measured using a UV-visible spectrophotometer (Elico S7-159) at weekly intervals to monitor the growth of the bacterial strains in synthetic media containing LDPE. Utilising the synthetic media, baseline correction was performed. Centrifugation at 12,000 rpm for 5 minutes at 4 °C was used to remove the cells from the liquid medium after the incubation process was complete. The dried cells were then weighed after drying, which was done.
2.7. Fourier transform-infrared spectroscopic analysis
FT-IR spectroscopy analysis (Perkin Elmer, Spectrum RX, USA) can be used to track the emergence or disappearance of functional groups as the degradation process progresses. At a resolution of 2 cm-1, spectra with a frequency range of 4000-400 cm-1 were employed. The following equations were used to compare the relative absorbance intensities of the ester carbonyl bond (1740 cm-1), keto carbonyl bond (1715 cm-1), terminal double bond (1650 cm-1) and internal double bond (908 cm-1) to those of the methylene bond at 1465 cm-1: , The internal double bond index (IDBI) is equal to I908/I1465 whereas the terminal vinyl bond index (TVBI) is equal to I1650 for keto carbonyl bonds (KCBI) and I1715/I1465 for ester carbonyl bonds (ECBI), the crystallinity (%) of the LDPE was determined and calculated: % of crystallinity = 100 [1 (Ia/1.233Ib)/1 + (Ia/Ib) 100], where Ia and Ib are absorbance values from the groups at 1474 and 1464 cm-1 or at 730 and 720 cm-1 separately.
2.8. Preliminary Studies for determining Optimum conditions
Selected recently obtained bacterial strains were cultured in nutrient broth by overnight incubation at 37°C and 120 rpm on an orbital shaker. This 24-hour-old culture was added to MSM media containing LDPE films in order to optimise the subsequent culture conditions for LDPE biodegradation.
Utilising MSM media, it was possible to optimise the physical and chemical parameters of LDPE breakdown by a few newly isolated bacteria. LDPE degradation tests were conducted using LDPE films as the only carbon source in a 250 ml shake flask with 100 ml of MSM media. enhancing the settings. To start the cultivation and degradation of LDPE, S6 & A3 strain was added to the medium. The chosen novel bacterial strains were raised in the identical incubation conditions, including 37 °C and 120 rpm.
2.9. Effect of parameters
Degradation was investigated in relation to contact time (24–120 hours), inoculum volume (1–6% v/v), temperature (32–42 0C), and pH (5.4–9.7). Using a micropipette, the prepared medium were carefully inoculated with the S-6 & A3 strain culture and autoclaved before being incubated at 37°C and 120rpm in an orbital shaking incubator. Every 24 hours, sampling was done, and data from spectrophotometric analysis were gathered.
3. Result and discussion
The current study focuses on the separation and degradation of LDPE, which is one of the most concerning issues facing humanity today since it results in the release of significant quantities of harmful compounds into the environment. In the current study, efforts were made to isolate LDPE-degrading organisms and to choose the strain that would degrade LDPE the fastest.
3.1. Isolation and characterization of bacterial strains
· Selective Isolation of LDPE Degrading Bacteria
Bacterial strains designated S1, S2, S3, S4, S5, and S6 were isolated from maritime soil, and bacterial strains named A1, A2, A3, A4, A5, and A6 were isolated from garden soil. Each of these bacterial strains had their growth monitored. S6 and A3 among the isolated isolates showed greater efficiency in LDPE degradation. Growth of various LDPE-degrading bacterial strains.
A soil sample was enhanced in sterile Mineral Salt Agar Medium (MSAM), with LDPE serving as the only carbon source of energy. The sample was further treated with LDPE films, which serve as an elective medium, to ensure that only LDPE resistant strains will survive in the medium. Microorganisms are therefore capable of degrading since they have successfully adapted to the current environment. As seen in Figure 2 and Figure 3 isolates were chosen, with S6 and A3 exhibiting the fastest growth rates among the tested strains.By using the streak plate method to culture the pure strains on Mineral salt agar medium (MSAM) plates, LDPE degradation-capable bacteria were chosen.
Figure 2
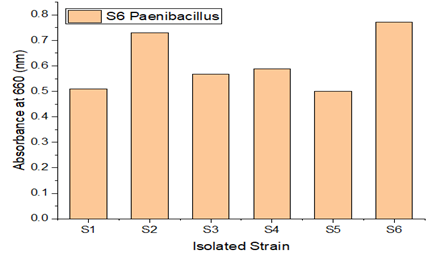
|
Figure 2 Growth of Different LDPE Degrading Different Bacterial Strains(S1-S6) |
Figure 3
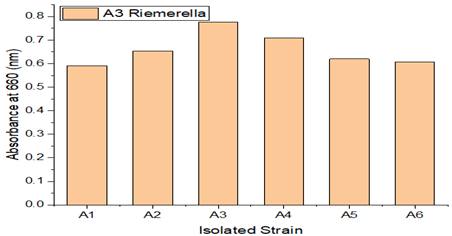
|
Figure 3 Growth of Different LDPE Degrading Different Bacterial Strains(A1-A6) |
3.2. Biolog GEN-III analysis
The relative capacity of strains S6 and A3 to utilise
substrate was activated using a suitable biology GENIII micro plate. The
relative ability of strains S6 and A3 to utilise substrate was activated using
a suitable biology GEN III microplate. After 24 hours of incubation, the
results indicated that the isolate significantly reacted with 59 of the 96
carbon substrates for S6 and 46 of the 96 carbon substrates for A3. According
to biology GEN III identification, the S6 plate's reaction profile matched Paenibacillus castanea
as closely as possible, while the A3 plate's response profile matched Riemerella anatipestifer
as closely as possible.
3.3. Optimization of physiological parameters in the given range
3.3.1. Effect of contact time
The recovered Paenibacillus
castanea and Riemerella
anatipestifer strains were investigated for their
ability to degrade LDPE over a range of time periods (Figure 4). It was found that
between 24 to 72 hours of contact time, the growth percentage grew more
rapidly. These findings indicate that a key element in the deterioration
process is the medium's contact time. After 72 hours, the rate of
disintegration usually starts to significantly decline. The effect of exposure
duration on the reaction of pure bacterial cultures and the microbial community
to LDPE toxicity was evaluated. It is indicated that the best results for the
degradation of LDPE would be obtained within 72 hours. Studies conducted by
Rani et al., 2021 and Khandare et al., 2021 both found similar reports Khandare
et al. (2021), Rani
et al. (2021).
Figure 4
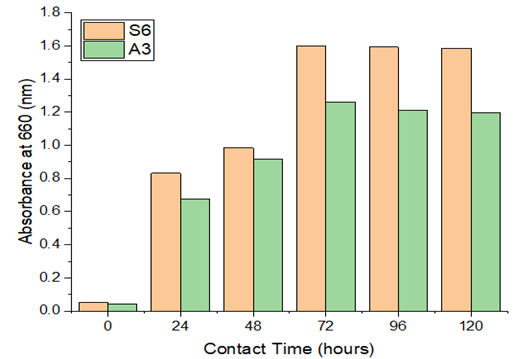
|
Figure 4 Effect of Contact Time& Absorbance |
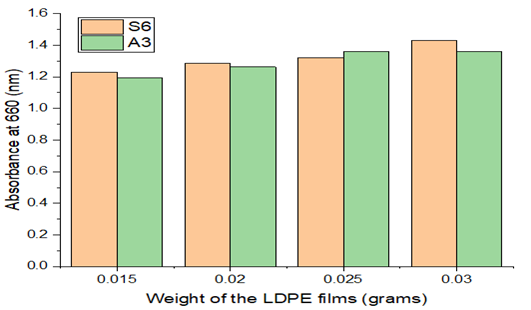
3.3.2. Effect of LDPE weight
Research on the impact of LDPE weight on microbial development was done through experiments. The results are displayed in Figure 5 of the data. In S6 plate Paenibacillus castanea and in A3 plate Riemerella anatipestifer strains grown microorganisms with consistent absorbance values up to weights of 0.030 and 0.042, respectively. According to the results, a stationary phase rise is seen as weight increases. The reports by Gajendiran et al., 2016, and Kyaw et al., 2012 are comparable Gajendiran et al. (2016), Kyaw et al. (2012).
Figure 5
|
Figure 5 Effect of LDPE Weight& Absorbance |
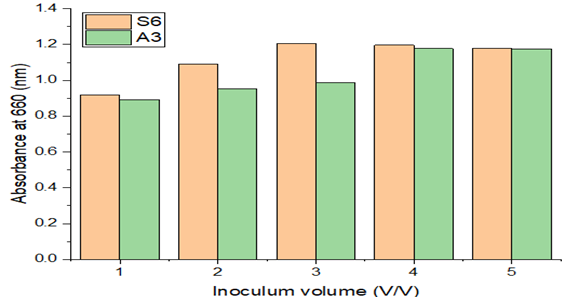
3.3.3. Effect of Inoculum volume
The growth of bacteria by isolated Paenibacillus castanea and Riemerella anatipestifer in S6 plate and A3 plate strains at various volumes of inoculums was investigated. It was found that increasing the inoculum volume from 1% (v/v) to 3% (v/v) enhanced the absorbance values from 0.891 to 1.179 for (S6) and 0.917 to 1.177 for (A3). These findings demonstrate that the medium's inoculum volume has a significant role in the degrading process.
The rate of absorbance tends to drop off quickly after inoculum volumes of 4% (v/v) for Riemerella anatipestifer and 3% (v/v) for Paenibacillus castanea the ideal inoculum value for both species in the specified range, as shown in Figure 6, was 3 & 4% v/v.
Figure 6
|
Figure 6 Effect of Inoculum Volume & Absorbance |
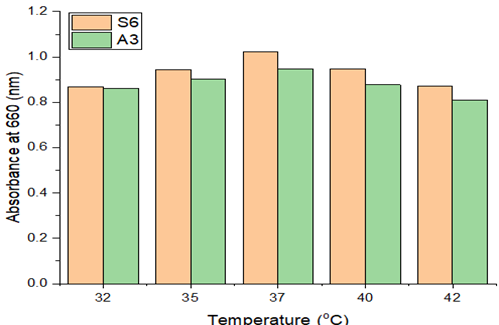
3.3.4. Effect of incubation temperature on LDPE degradation
Environmental temperature and microbial activity are directly correlated in microorganisms because the microbial cell adapts to changes in temperature using biochemical or enzymatic mechanisms. At various temperatures (32 to 42°C), experiments were conducted to investigate the impact of temperature on the percentage of LDPE deterioration during a continuous 72-hour period (Figure 7). The proliferation of microorganisms was seen to rise when the incubation temperature rose from 32 to 37°C. Cells may become metabolically active and able to create the necessary enzymes for breakdown. The capacity of the bacterial culture to degrade was drastically reduced when the temperature increased more. This might have happened as a result of the negative impact that high temperatures have on enzyme activities. It was thought that abrupt exposure to temperatures above 37°C would be harmful to the bacterial enzymes. On the other side, lower temperatures are expected to slow down bacterial activity. Similar reports were investigated by Raaman et al. (2012), Yoon et al. (2012).
Figure 7
|
Figure 7 Effect of Temperature |
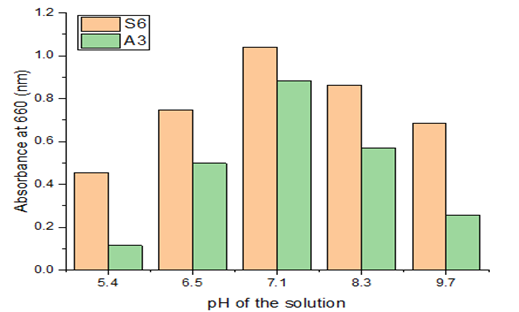
3.3.5. Effect of pH
The growth of the strain that breaks down LDPE at various pH levels was investigated. It was found that the development of the LDPE resistant strain, Paenibacillus castanea was raised from 0.685 to 0.881 when the pH was raised from 5.4 to 7.1. The growth of the LDPE resistance strain increased from 0.455 to 1.040 with a pH increase from 5.5 to 7.5 and dropped from 1.040 to 0.257 with a pH rise from 7.1 to 9.7 for the Riemerella anatipestifer strain. According to these findings, a medium's pH plays a significant role in the degradation process. The bacterium displayed its maximal growth potential at 7.1, as seen in the Figure 8. The ability of bacteria to break down material is a prerequisite for cell growth and active metabolism in culture. The outcomes indicate that the organism can actively destroy the LDPE under neutral conditions. By means of this study, it is clear that the strain grows actively at pH-7.1. Both acidic and alkaline pH inhibited development. Even proteins that could harm the microbe were naturized as a result of pH changes.
Figure 8
|
Figure 8 Effect of Ph& Absorbance |
3.3.6. Dry weight determination of recovered LDPE
Every seven days, the remaining LDPE strips were removed from the culture plates. The mass of bacteria that was stuck to the LDPE surface was first cleaned with ethanol for 30 minutes, then with distilled water. The cleaned LDPE particles were dried by air before being weighed. Utilising the % of LDPE degradation formula, the weight loss of the LDPEs was determined. Paenibacillus castanea and Riemerella anatipestifer were both shown to have a potency of 7.30% and 5.40%, respectively, for the degradation of LDPE over the course of 35 days (Figure 9). In line with the research done by Tokiwa et al. (2009), Abraham et al. (2017).
Calculation:
% LDPE Degradation = ![]()
Figure 9
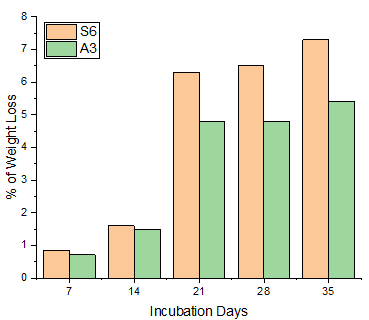
|
Figure 9 Effect of Incubation Days & Weight Loss |
3.4. FTIR analysis
The control polymer film spectra displayed several peaks illustrating the complex nature of the LDPE (polymer film not exposed to microorganisms) Figuratively 10. When test samples were analysed after being incubated with microorganisms, S6, there was a variance in the intensity of bands in various places.
The distinctive absorption bands for the control spectrum were assigned at 719 cm-1 (C-H bendmono), 1,472 cm-1 (C=C stretch), 2,660 cm-1 (CHO stretch), and 2,919, 2,850 cm-1 (both owing to C-H stretch).The peak at 2,660 cm-1 corresponds to CHO stretching vibration that has been disappeared in case of Paenibacillus castanea while new band has been observed at 939 cm-1 (O–H bend) which supports the depolymerization activity of the microbial isolates. After microbial treatment, the strong absorption maxima at 719 and 1,472 cm-1 became weaker. In addition, peaks at 2,919 and 2,850 cm-1 became sharper in the treated sample than the control one, here also the same microbial activity pattern were seen. The change in the peak values of almost all functional groups supporting the conformational change on polymer surface. This may indicate that in our study, the degradation of LDPE has a high product selectivity Kunlere et al. (2019), Deepa (2019), Nanthini et al. (2021), Deepika & Jaya (2015).
Figure 10
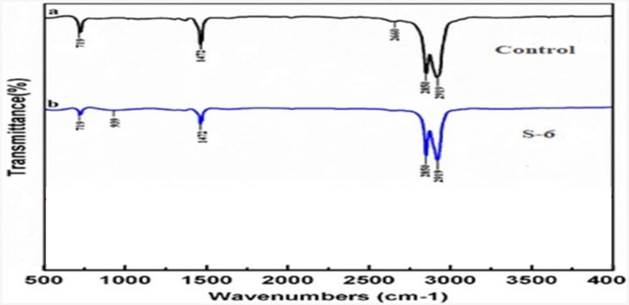
|
Figure 10 FTIR of Control (a) Treated with S6 |
4. Conclusion
Due to the fact that it doesn't create any harmful byproducts, biodegradation is one of the least expensive processes. The prospect of total deterioration and reduced costs make this procedure commonly selected. With LDPE serving as the only carbon supply and energy, the growth and LDPE biodegradation study was conducted in MSAM. The ability of each strain to degrade LDPE was initially assessed. Paenibacillus castanea & Riemerella anatipestifier are the LDPE-degrading bacteria, according to the Gen III Microlog report's analysis of the isolated strains S6 & A3. According to studies, Paenibacillus castanea (S6) and Riemerella anatipestifier (A3) could degrade LDPE by 7.30% and 5.40%, respectively, given the best growing conditions during 35 days duration. Paenibacillus castanea is more effective at degrading LDPE than Riemerella anatipestifier, according to the percentage weight loss. For Paenibacillus castanea the formation of new functional groups and reduction in the absorption characteristics of the peaks detected by FTIR spectra.
CONFLICT OF INTERESTS
The authors state that they do not have any known competing financial interests or personal ties that could appear to have influenced the work disclosed in this study.
ACKNOWLEDGMENTS
The authors would like to express their gratitude to the Department of Chemical Engineering at Andhra University in Visakhapatnam, Andhra Pradesh, India.
REFERENCES
Abraham, J., Ghosh, E., Mukherjee, P., & Gajendiran, A. (2017). Microbial Degradation of Low Density Polyethylene. Environmental Progress & Sustainable Energy, 36(1), 147-154. https://doi.org/10.1002/ep.12467.
Alshehrei, F. (2017). Biodegradation of Synthetic and Natural Plastic by Microorganisms. Journal of Applied & Environmental Microbiology, 5, 8-19. https://doi.org/10.12691/jaem-5-1-2.
Chatterjee, S., Roy, B., Roy, D., and Banerjee, R. (2010). Enzyme-Mediated Biodegradation Of Heat Treated Commercial Polyethylene By Staphylococcal Spp. Polym. Degrad. Stab. 95(2), 195-200. https://doi.org/10.1016/j.polymdegradstab.2009.11.025.
Danso, D., Chow, J., and Streita, W.R. (2019). Plastics : Environmental and Biotechnological Perspectives on Microbial Degradation. Applied and Environmental Microbiology, 85(19). https://doi.org/10.1128/AEM.01095-19.
Das, M. P., and Kumar, S. (2014). Microbial Deterioration of Low Density Polyethylene by Aspergillus and Fusarium Sp. Int J Chem Tech Res, 6(1), 299-305.
Das, M.P., and Kumar, S. (2015). An Approach to Low-Density Polyethylene Biodegradation by Bacillus Amyloliquefaciens. 3 Biotech, 5, 81-6. https://doi.org/10.1007/S13205-014-0205-1.
Deepa, D. (2019). Biodegradation of Low Density Polyethylene by Selected Bacillus sp. Gazi University Journal of Science, 32(3), 802-813. https://doi.org/10.35378/gujs.496392.
Deepika, S., & Jaya, M. R. (2015). Biodegradation of Low Density Polyethylene by Microorganisms from Garbage Soil. J Exp Biol Agric Sci, 3, 1-5.
Dey, A. S., Bose, H., Mohapatra, B., and Sar, P. (2020). Biodegradation of Unpretreated Low-Density Polyethylene (LDPE) by Stenotrophomonas sp. And Achromobacter sp., Isolated from Waste Dumpsite and Drilling Fluid. Frontiers in Microbiology, 11. https://doi.org/10.3389/fmicb.2020.603210.
Fei, Y., Huang, S., Zhang, H., Tong, Y., Wen, D., Xia, X., Wang, H., Luo, Y., and Barceló, D. (2020). Response of Soil Enzyme Activities and Bacterial Communities to the Accumulation of Microplastics in an Acid Cropped Soil. Science of the Total Environment, 707. https://doi.org/10.1016/j.scitotenv.2019.135634.
Francis, V., Raghul, S.S., Sarita, G.B., and Eby, T.T. (2010). Microbial Degradation Studies on Linear Low-Density Poly (Ethylene)-Poly (Vinyl Alcohol) Blends Using Vibrio Sp. International Conference on Advances in Polymer Technology, 26-27.
Gajendiran, A., Krishnamoorthy, S., and Abraham, J. (2016). Microbial Degradation of Low-Density Polyethylene (LDPE) by Aspergillus Clavatus Strain JASK1 Isolated From Landfill Soil. 3 Biotech, 6, 1-6. https://doi.org/10.1007/S13205-016-0394-X.
Gupta, K.K., and Devi, D. (2020). Characteristics Investigation
on Biofilm Formation and Biodegradation Activities of Pseudomonas Aeruginosa
Strain ISJ14 Colonizing Low Density Polyethylene (LDPE) Surface. Heliyon. https://doi.org/10.1016/j.heliyon.2020.e04398.
Huang, Y., Zhao, Y., Wang, J., Zhang, M., Jia, W., and Qin, X. (2019).
LDPE Microplastic Films Alter Microbial Community Composition And Enzymatic
Activities In Soil. Environmental Pollution. 254(Pt A). https://doi.org/10.1016/j.envpol.2019.112983.
Khandare, S. D., Chaudhary, D. R., & Jha, B. (2021). Marine Bacterial Biodegradation of Low-Density Polyethylene (LDPE) plastic. Biodegradation, 32(2), 127-143. https://doi.org/10.1007/s10532-021-09927-0.
Kunlere, I. O., Fagade, O. E., & Nwadike, B. I. (2019). Biodegradation of Low Density Polyethylene (LDPE) by Certain Indigenous Bacteria and Fungi. International Journal of Environmental Studies, 76(3), 428-440. https://doi.org/10.1080/00207233.2019.1579586.
Kyaw, B.M., Champakalakshmi, R., Sakharkar, M.K., Lim, C.S., and Sakharkar, K.R. (2012). Biodegradation of Low Density Polythene (LDPE) by Pseudomonas Species. Indian J Microbiol, 52, 411-9. https://doi.org/10.1007/S12088-012-0250-6.
Li, Z., Wei, R., Gao, M., Ren, Y., Yu, B., Nie, K., Xu, H., and Liu, L. (2020). Biodegradation of Low-Density Polyethylene by Microbulbifer Hydrolyticus IRE-31. Journal of Environmental Management, 263. https://doi.org/10.1016/J.JENVMAN.2020.110402.
Muhonja, C.N., Makonde, H., Magoma, G., and Imbuga, M. (2018). Biodegradability of Polyethylene by Bacteria and Fungi from Dandora Dumpsite Nairobi-Kenya. PLoS One, 13. https://doi.org/10.1371/JOURNAL.PONE.0198446.
Nanthini, D. K., Raju, P., Santhanam, P., Dinesh, S., Krishnaveni, N., Roopavathy, J., & Perumal, P. (2021). Biodegradation of Low-Density Polyethylene and Polypropylene by Microbes Isolated from Vaigai River, Madurai, India. Archives of Microbiology, 203, 6253-6265. https://doi.org/10.1007/s00203-021-02592-0.
Okunola, A. A., Kehinde, I. O., Oluwaseun, A., and Olufiropo, E. A. (2019). Public and Environmental Health Effects of Plastic Wastes Disposal: A Review. Journal of Toxicology and Risk Assessment. https://doi.org/10.23937/2572-4061.1510021.
Plastic Europe, (2018). Plastic- the Facts 2018.
Raaman, N., Rajitha, N., Jayshree, A., & Jegadeesh, R. (2012). Biodegradation of Plastic by Aspergillus spp. Isolated from Polythene Polluted Sites Around Chennai. J. Acad. Indus. Res, 1(6), 313-316.
Raddadi, N., Fava, F. (2019). Biodegradation of Oil-Based Plastics in the Environment : Existing Knowledge and Needs of Research and Innovation. Science of the Total Environment, 679, 148-58. https://doi.org/10.1016/j.scitotenv.2019.04.419.
Rani, R., Singh, N. P., and Santal, A. R. (2021). Isolation, Characterization and Optimization of Bacterial Isolate Sarr1 for Biodegradation of Pretreated Low Density Polyethylene. Journal of Applied and Natural Science, 13(2), 561-570. https://doi.org/10.31018/jans.v13i2.2663.
Ru, J., Huo, Y., and Yang, Y. (2020). Microbial Degradation and Valorization of Plastic Wastes. Front Microbiol, 11. https://doi.org/10.3389/fmicb.2020.00442.
Sen, S. K., and Raut, S. (2015). Microbial Degradation of Low Density Polyethylene (LDPE) : à Review. Journal of Environmental Chemical Engineering, 3(1), 462-473. https://doi.org/10.1016/j.jece.2015.01.003.
Tokiwa, Y., Calabia, B. P., Ugwu, C. U., & Aiba, S. (2009). Biodegradability of Plastics. International Journal of Molecular Sciences, 10(9), 3722-3742. https://doi.org/10.3390/ijms10093722.
Vatseldutt, S. A. (2014). "Isolation and Characterization of Polythene Degrading Bacteria from Polythene Dumped Garbage". Int J Pharm, 25(2), 205-206.
Yoon, M. G., Jeon, H. J., & Kim, M. N. (2012). Biodegradation of Polyethylene by a Soil Bacterium and AlkB Cloned Recombinant Cell. Journal of Bioremediation & Biodegradation, 3(4), 1-8. https://doi.org/10.4172/2155-6199.1000145.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2023. All Rights Reserved.