
RESISTANCE OF Klebsiella pneumoniae TO ANTIBIOTICS OF CAT TREATED AT ANIMAL CLINIC
Safika 1 ![]()
![]() ,
Lintang Wulandari 2
,
Lintang Wulandari 2![]() , Arief
Purwo Mihardi 3
, Arief
Purwo Mihardi 3![]() , Usamah Afif 1
, Usamah Afif 1![]() , Agustin Indrawati 1
, Agustin Indrawati 1![]() , Rahmat Hidayat
1
, Rahmat Hidayat
1![]() , Titiek Sunartatie
1
, Titiek Sunartatie
1![]()
1 Division of Medical Microbiology, School of Veterinary Medicine, and Biomedical Sciences, IPB University, Bogor, Indonesia
2 Student of School of Veterinary
Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia
3 Division of Internal Medicine,
School of Veterinary Medicine, and Biomedical Sciences, IPB University, Indonesia
|
|
ABSTRACT |
||
|
The antibiotic
resistance of Klebsiella pneumoniae has become a global health concern,
leading in a reduction in the efficacy of various medications. This study
seeks to determine the antibiotic resistance of K. pneumoniae isolated from
cats hospitalized in Depok veterinary clinics. Surveys, sample collection,
isolation, identification, and antibiotic susceptibility testing comprised
the research technique. Seven K. pneumoniae isolates were discovered. There
were seven cefotaxime resistant isolates, three amoxicillin resistant
isolates, one gentamicin resistant isolate, and one enrofloxacin resistant
strain. At the same concentration, one isolate shown intermediate
susceptibility to amoxicillin, enrofloxacin, and doxycycline. Sensitivity was
demonstrated by six gentamicin isolates, six doxycycline isolates, five
enrofloxacin isolates, and three amoxicillin isolates. This information
should help veterinarians choose the best efficient antibiotic to treat
infections caused by these bacteria. |
|||
|
Received 03 November 2022 Accepted 02 December 2022 Published 31 December 2022 Corresponding Author Safika, fikakhan@yahoo.com DOI10.29121/granthaalayah.v10.i12.2022.4932 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2022 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Animal Clinic, Antibiotic, Antibiotic
Resistance, Klebsiella Pneumoniae |
|||
1. INTRODUCTION
Cats are little Felidae carnivores that have been domesticated for thousands of years and are quite close to humans due to their adaptability. This process of domestication can make cats prone to disease. Infections of the respiratory tract are common in cats Epstein et al. (2010) and are associated with substantial morbidity and death Schulz et al. (2006).
Cats' lower respiratory tract disease is typically caused by infectious agents (viruses, bacteria, fungi, and parasites); however, heart disease, neoplasia, and trauma can also cause respiratory disease. Most feline respiratory illnesses are caused by pneumonia Bart, et al. (2000). Due to a weakened immune system, pathogenic bacteria or the multiplication of normal bacteria can cause bacterial respiratory tract disorders Schulz et al. (2006). Common bacteria that cause pneumonia in cats are Pasteurella multocida, Escherichia coli, Streptococcus canis, Bordetella bronchiseptica, and Klebsiella pneumoniae Henik and Yeager (1994). Klebsiella pneumoniae is the most prevalent cause of nosocomial infections Podschun and Ullmann (1998).
Upper respiratory tract disease in cats is characterized by nasal discharge that ranges from serous to mucopurulent, sneezing, epistaxis, and conjunctivitis. Clinical signs may be acute (lasting less than 10 days) or chronic (lasting more than 10 days). Cats with clinical indications of upper respiratory tract disease caused by one or more pathogenic viral, bacterial, or fungal species are diagnosed with an upper respiratory infection Lappin et al. (2017). Oropharyngeal swabs from cats with respiratory tract infections can be utilized to isolate numerous types of bacteria, including Klebsiella spp. at a rate of 3.0%. Adler et al. (2007).
Acute respiratory infections can be treated with antibiotics. Long-term, non-adhered use and divergence from dose standards are the root causes of antibiotic resistance. Numerous conventional antibiotics have lost efficacy because of bacterial drug resistance, which has become a global health concern. Numerous research has been published on the antibiotic resistance of these bacteria in humans. In 2003, the National Nosocomial Infections Surveillance revealed a 47% increase in resistance to third generation cephalosporins among Klebsiella pneumoniae isolates from intensive care unit patients, compared to the five-year average resistance rate National Nosocomial Infections Surveillance System [NNIS] (2004). The extended-spectrum beta-lactamases (ESBL) produced by these microbes provide resistance to penicillin, cephalosporins, ceftazidime, cefoxitin, ceftobiprole, and aztreonam Peterson et al. (2003). Resistance to tetracyclines, trimethoprim-sulfamethoxazole, quinolones, and aminoglycosides has been reported to grow in human nosocomial infections Bouza and Cercenado (2002). Schulz et al. (2006) assessed the proportion of sensitivity of different antibiotics, including enrofloxacin (one hundred percent), gentamicin (ninety percent), and amoxicillin (sixty-six percent), against Enterobacter isolated from cats with respiratory illnesses. Klebsiella pneumoniae isolates displayed the highest levels of resistance to amoxicillin-clavulanate (74.3%, n = 78/105), doxycycline (52.4%, n = 55/105) and trimethoprim sulfamethoxazole (57.1%, n = 60/105), respectively Li, et al. (2021).
The majority of Klebsiella pneumoniae infections cannot be effectively treated in the field. Klebsiella pneumoniae in cats was resistant to multiple medications, including penicillin, cephalosporins, ceftazidime, cefoxitin, and ceftazidol, according to multiple investigations. This study aims to evaluate the Klebsiella pneumoniae resistance to amoxicillin, enrofloxacin, gentamicin, cefotaxime, and doxycycline identified in cats hospitalized in a clinic in Depok City, Indonesia.
2. MATERIALS AND METHODS
2.1. QUESTIONNAIRE
The questionnaire was used to collect information about
the clinics and inpatient cats. This poll is for Veterinarians who work at
animal clinics in Depok, West Java
Indonesia. The questionnaire consists of three sections: the first
portion covers the clinic's identity, the second section contains clinical
activities, including data on inpatient cats used in the study, and the third
section contains information on the antibiotics used in the clinic. The
antibiotics utilized in the study will be chosen based on the antibiotic data
collected for the five antibiotics used most frequently in each clinic.
2.2. SAMPLE COLLECTION
Collecting oropharyngeal swabs from cats hospitalized in the Depok City clinic West Java Indonesia. The oropharyngeal swab sample was placed in a sterile tube with a peptone water buffer containing 0.1% peptone and then placed in a cool box. After collecting all samples, they were taken to the laboratory and placed in the refrigerator for identification and testing of their resistance.
2.3. ISOLATION AND IDENTIFICATION OF KLEBSIELLA PNEUMONIAE
Mac Conkey Agar (MCA) medium was
used to cultivate swab samples for 24 hours at 37 °C. The colonies generated by
Klebsiella bacteria are spherical, smooth, convex, pink, and mucoid. On
Tryptic Soy Agar (TSA), single
colonies suspected of belonging to the genus Klebsiella were subcultures
for 24 hours at 37 °C.
The TSA obtained isolates were subsequently characterized. Gram staining was the initial method of identification. Using Gram staining, the morphology of bacterial isolates was determined. Red coccobacillus appears as a tiny form in Klebsiella bacteria. A biochemical test is the following step in the identifying procedure. The oxidase test, the Triple Sugar Iron Agar (TSIA) test, the urease test, and the IMViC test were performed as biochemical analyses. Multiple tests comprise the IMVIC exam, including the Sulfide Indole Motility (SIM) test, Methyl Red-Voges Proskauer test, and Simmon's Citrate test. Biochemical tests, such as Triple Sugar Iron Agar (TSIA), the urease test, and the IMViC test, were incubated twice for 24 hours at 37 °C. As shown by the absence of a purple color shift in the bacterial isolates, the oxidase test for Klebsiella bacteria produced a negative result. During the TSIA test, Klebsiella pneumoniae will not release H2S gas or make the puncture site black during the TSIA test. The Indole Motility and Voges Proskauer tests were negative, however the Methyl-Red, urease, and Klebsiella pneumoniae Simmon's Citrate tests were positive.
The fermentation test is the conclusive way for identifying Klebsiella. For the carbohydrate fermentation test, glucose, lactose, sucrose, maltose, mannitol, and dulcitol were employed as sugars. At 37 °C, the carbohydrate fermentation test was incubated for 24 hours. The results of the Klebsiella pneumoniae carbohydrate fermentation test was positive.
2.4. ANTIBIOTIC SENSITIVITY TESTING
Kirby-Bauer disk diffusion and Mueller-Hinton agar are applied for the antibiotic sensitivity test. The sample of Klebsiella pneumoniae bacteria was originally cultivated on TSA media and then incubated at 37°C for 24 hours. Using loops, several colonies grown on TSA media were transferred to a test tube containing 5 mL of physiological NaCl. The mixture was then homogenized with a vortex, and the turbidity was standardized to a solution of 0.5 McFarland. Using a micropipette, 0.5 mL of the suspension mixture is dripped into MHA medium, and the suspension is subsequently flattened using a stir bar. After equally spreading the suspension on the MHA medium, the antibiotic disc paper was placed on top of the Mueller Hinton Agar (MHA) and incubated at 37 °C for 24 hours prior to measurement. The antibiotic inhibition zone was measured by measuring the diameter of the inhibition space with a vernier caliper. The antibiotic sensitivity test was performed twice at the same time. The diameter of the obtained antibiotic inhibition zone was afterwards compared to the diameter standard inhibition zone of the CLSI (2021).
3. RESULTS AND DISCUSSION
Seven veterinary clinics in the city of Depok contributed a total of 20 oropharyngeal swabs from cats. Since the number of feline patients at each clinic varies, so does the quantity of samples collect. Priority is given to collecting samples from cats with respiratory difficulties, but if none are available, samples are collected from cats with diseases other than respiratory tract disorders. Figure 2 displays the proportion and kind of disease observed in the 20 oropharyngeal swab samples taken for this study from cats. Forty percent (n = 8/20) of the sampled cats had respiratory tract problems. Lee et al. (2021) found that Klebsiella is present in the nasal cavity at a rate of 14%, whereas Adler et al. (2007) found that oropharyngeal swabs may include up to 3%.
The findings of the questionnaire are displayed in Figure 1 and Figure 2. The data received from the questionnaire pertain to hospitalized cats and the antibiotics used in the clinic. Figure 3 displays the most frequently utilized antibiotics in the seven clinics from which samples were gathered. Antibiotics used in this trial were amoxicillin, cefotaxime, gentamicin, enrofloxacin, and doxycycline. The selection of antibiotics was based on the findings of a questionnaire, namely the top five antibiotics used most in clinics, considering the availability of laboratory materials.
Figure 1
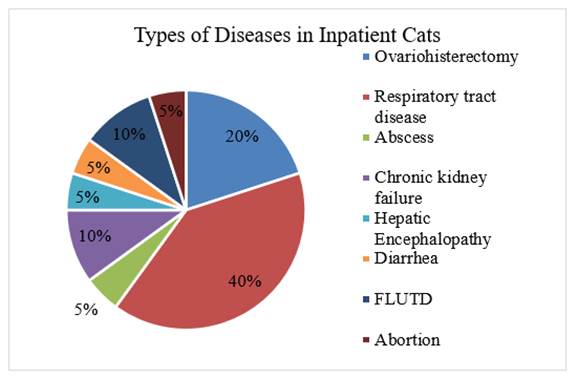
|
Figure 1 Types of Feline Disorders Treated at the Veterinary Hospital in Depok, Indonesia |
Mac Conkey Agar is a selective medium that contains bile salts and is useful for isolating Enterobacteriaceae and other Gram-negative bacteria. Pink mucoid colonies were produced because of the oropharyngeal swab isolation that occurred on this medium. This medium makes it possible to isolate lactose-fermenting bacteria from those that cannot ferment lactose. This medium comprises lactose and a pH indicator of neutral red. If the bacteria growing on the medium are digesting lactose, the acids by product will tint the medium and colonies pink. Mucoid colonies are a distinctive characteristic of Klebsiella and Enterobacter species Quinn et al. (2011).
Figure 2
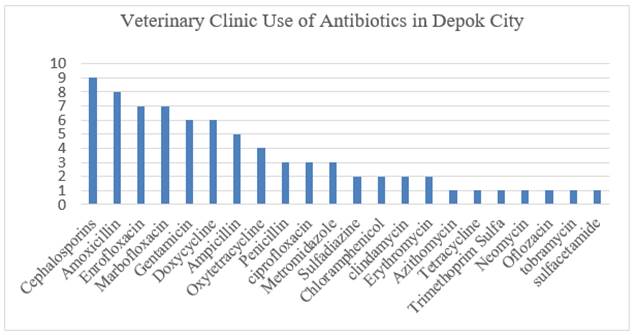
|
Figure 2 Graph of Antibiotic Use at the Depok City Veterinary Clinic |
On microscopic examination with Gram stain, Klebsiella pneumoniae colonies displayed the characteristic short, red bacilli of Gram-negative bacteria. Due to the presence of lipids and polysaccharides on the bacterial cell wall, Gram-negative bacteria have a red color when viewed under the microscope. When alcohol and Crystal violet dye are added, the layer degrades and separates from the peptidoglycan thin layer, allowing the second, red-colored safranin dye to adhere to the bacteria Cappuccino and Sherman. (2014). The oxidase test yielded a negative result, which was followed by a biochemical analysis.
The biochemical examination includes IMViC, urease, TSIA, and fermentation of carbohydrates assays. The IMViC test, which consists of Indole, Methyl red, Voges Proskauer, and Simmon's Citrate, revealed distinct results for each of the investigated isolates. The findings of the indole test were negative for all the investigated isolates. The indole test measures the synthesis of indole from the decomposition of the amino acid tryptophan. The tryptophanase enzyme will hydrolyze the tryptophan found in the indole test medium. This tryptophanase enzyme will produce three end products, with indole being one of them. A positive indole test will provide a red hue, whereas a negative result will yield a yellow hue. K. pneumoniae is a bacteria whose indole test findings are negative. The Methyl red test results for some isolates were inconclusive, while others were positive. The methyl red test is used to evaluate the production of strong acids during the process of glucose fermentation. In this test, K. pneumoniae will produce a red color Cappuccino and Sherman (2014). All isolates that were subjected to the Voges-Proskauer test were negative. The Voges-Proskauer test is used to identify bacteria that can produce and control acid and ferment glucose, as well as to discover bacteria that produce neutral products (acetyl carbinol or acetoin) from glucose fermentation Cappuccino and Sherman (2014). According to Cowan and Steel (1993) identification key, the Methyl red test and the Voges-Proskauer test will provide positive and negative results for K. pneumoniae isolates.
The outcomes of citrate and urea tests on individual isolates varied. The results of the Simmon's Citrate Test are indicated by a change from green to blue in the color of the indicator, indicating a favorable result. This change suggests that during the growth of bacteria in citrate medium, the bacteria produce an alkaline state and utilize citrate as an energy source. K. pneumoniae responded positively to the citrate test. The urea test is used to determine whether bacteria can produce the enzyme urease K. pneumoniae will yield a positive result in this test, evidenced by a pink change in the medium's hue Cappuccino and Sherman (2014).
Triple Sugar Iron Agar (TSIA) consists of 0.1% glucose, 1.0% lactose, and 1.0% sucrose in addition to components that suggest H2S production. Phenol red is utilized to indicate variations in pH. (Red at a pH of 8.2 and yellow at 6.4) According to Quinn et al. (2011), the TSIA test for K. pneumoniae bacteria released gas, did not produce H2S, and the slender and blunt sections were yellow. The results of the TSIA test confirm these findings. Biochemical reactions in K. pneumoniae isolates were able to ferment glucose, lactose, maltose, mannitol, and sucrose, according to Cappuccino and Sherman (2014). The capacity of K. pneumoniae to ferment carbohydrates reveals that the bacteria can produce acid, as evidenced by the yellowing of the medium and the presence of gas in the Durham tube when the indicator is added.
Following completion of all identifying procedures, seven K. pneumoniae isolates were
recovered. According to the results of the inpatient cat data questionnaire, up
to seven bacterial isolates were obtained from cats with various diseases. Five
of the isolates originated from hospitalized cats with respiratory disease, one
from post-abortion care, and one from chronic kidney failure. K. pneumoniae is a bacterium
that can cause nosocomial infections Foster et al. (2004). Nosocomial infections
are infections acquired by hospitalized persons. In veterinary medicine, the
most often identified nosocomial infections are urinary tract infections,
pneumonia, bloodstream infections, surgical site infections, and infectious diarrhea. The prevalence of this nosocomial infection
appears to increase as the number of animal clinics and hospitals that provide
intensive care grows. Lengthy hospital stays and the use of invasive gadgets
and procedures can increase the likelihood of contracting this infection Hakvoort et al. (2020).
Figure 3
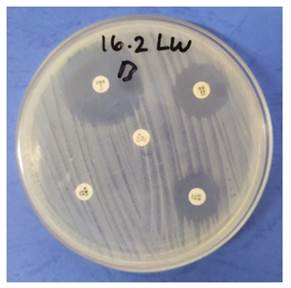
|
Figure 3 The Inhibition Zone Measurement for the K. Pneumoniae Sensitivity Test to Amoxicillin, Cefotaxime, Gentamicin, Enrofloxacin, And Doxycycline |
K.
pneumoniae identified isolates are subsequently examined for their
susceptibility to various drugs. Using the Kirby-Bauer disk diffusion method
and Mueller-Hinton agar, the sensitivity test is undertaken. As depicted in Figure 3, the findings of this
test demonstrate that an inhibitory zone of bacterial growth has formed around the
antibiotic disc paper. This inhibition zone is produced when the agar plate is
infected with a standard inoculum of the microorganism being evaluated and the
antibiotic disc containing the antibiotic compound to be evaluated is placed on
top. The diameter of the growth inhibitory zone is then determined Balouiri et al. (2016). The findings of
measuring the diameter of the inhibitory zone are presented in Table 1. Each individual
isolate measures different types of antibiotics differently. The percentage
findings are provided in Table 2 based on the results
of measuring the diameter of the inhibition zone and comparing it to the
Clinical and Laboratory Standards Institute's standard diameter of the
inhibition zone.
Table 1
|
Table 1 The Average Results of Antibiotic Sensitivity Tests for K. Pneumoniae |
|||||
|
Bakteria Isolate |
Average
Antibiotic Inhibition Zone |
||||
|
|
AML |
CTX |
CN |
ENR |
DO |
|
1 |
9.55 |
0 |
17.65 |
26.32 |
18.65 |
|
2 |
20.25 |
0 |
23.05 |
28.5 |
30.67 |
|
3 |
15.25 |
0 |
18.05 |
27.25 |
27.27 |
|
4 |
0 |
0 |
17.3 |
20.5 |
13.25 |
|
5 |
0 |
0 |
17.3 |
29.12 |
20.70 |
|
6 |
29.4 |
13.4 |
7 |
9.67 |
14.45 |
|
7 |
19.5 |
0 |
20.45 |
28.5 |
22.10 |
|
AML (Amoxicillin), CTX (Cefotaxime), CN (Gentamycin), ENR (Enrofloxacin), DO (Doxycycline) |
|||||
The amoxicillin sensitivity test indicated an equal number of resistant and sensitive isolates, as well as one isolate with intermediate results. Three bacterial isolates (42.85%) were determined to be resistant; three bacterial isolates (42.85%) were determined to be sensitive, and one bacterial isolate (14.28%) was determined to be intermediate. Intermediate level of difficulty in the intermediate group, a higher dose can inhibit the growth of the isolate James et al. (2015). This renders Klebsiella pneumoniae resistant to amoxicillin, as higher doses are necessary to inhibit bacterial growth. Amoxicillin is the antibiotic of choice for treating acute and chronic upper respiratory infections, according to Lappin et al. (2017). Amoxicillin is a penicillin-derived beta-lactam derivative. By interacting with cell receptors known as penicillin-binding proteins, beta-lactam antibiotics restrict the synthesis of cell walls. In addition, this category of antibiotics can increase autolysin activity, leading to cell death Quinn et al. (2011).
Table 2
|
Table 2 Proportion of Klebsiella Pneumoniae Susceptible to Antibiotics Based on Test Results |
||||||
|
Antibiotics |
Type |
|||||
|
|
Susceptible |
Intermediates |
Resistance |
|||
|
|
Total |
% |
Total |
% |
Total |
% |
|
Amoxicillin |
3 |
42.85% |
1 |
14.28% |
3 |
42.85% |
|
Cefotaxime |
- |
- |
- |
- |
7 |
100% |
|
Gentamicin |
6 |
85.71% |
- |
- |
1 |
14.28% |
|
Enrofloxacin |
5 |
71.42% |
1 |
14.28% |
1 |
14.28% |
|
Doxycycline |
6 |
85.71% |
1 |
14.28% |
- |
- |
According to the results of the sensitivity test, all seven Klebsiella pneumoniae isolates were resistant to cefotaxime. To a lesser extent than 50%, Lee et al. (2021) discovered that K. pneumoniae species were resistant to cefotaxime. Because bacteria acquire beta-lactamase enzymes, resistance to cefotaxime medications is feasible. Because beta-lactamase enzymes can splinter beta-lactam rings and render antibiotics ineffective, bacteria that develop these enzymes will be resistant to beta-lactam antibiotics Quinn et al. (2011). According to Lee et al. (2021) 16 K. pneumoniae strains possess genes for extended-spectrum b-lactamases (ESBL). Changes or mutations in the Penicillin Binding Protein (PBP) protein-coding gene are one of the reasons of beta-lactam resistance. The binding of beta-lactam antibiotics to PBP inhibits the development of bacterial cell wall, resulting in cell lysis Brisse and Duijkeren (2005).
The results of this study, six K. pneumoniae isolates were susceptible to the antibiotic gentamicin, whereas one was resistant. Schulz et al. (2006) determined that gentamicin was 90 percent effective against Enterobacteriaceae. This class of aminoglycoside antibiotics will block the function of the 30S ribosome, hence inhibiting protein synthesis. In addition to being efficient against aerobic Gram-negative bacilli and S. aureus, this class of antibiotics is extraordinarily strong against Enterobacteriaceae, P. aeruginosa and Acinetobacter spp James and John, (2015).
Five Klebsiella pneumoniae isolates (71.42%) were sensitive to the antibiotic enrofloxacin, one isolate was intermediate (14.28%), and one strain was resistant (14.28%) according to the sensitivity test. The fluoroquinolone drug enrofloxacin has antibacterial characteristics. This class of antibiotics inhibits DNA gyrase, hence limiting nucleic acid synthesis Quinn et al. (2011). This medication has a broad spectrum against Gram-negative bacteria and Mycoplasma spp., and it is commonly used as a parenteral treatment for animals. Other fluoroquinolone veterinary drugs, such as enrofloxacin, marbofloxacin, and orbifloxacin, have been used to treat upper respiratory tract infections in cats caused by bacteria. Intravenously administering enrofloxacin and broad-spectrum antibiotics against Gram-positive and anaerobic bacteria to dogs or cats with pneumonia and sepsis is possible Lappin et al. (2017).
Six isolates (85.71%) were susceptible to the drug doxycycline, one was intermediate, and none were resistant. This class of tetracycline antibiotics suppresses the synthesis of proteins. Doxycycline will reversibly bind to the 30S subunit and inhibit aminoacyl-tRNA binding to the bacterial ribosome. In mitochondria, binding to the 70S ribosome further inhibits ribosome activity. This bacteriostatic drug will enter the bacterial cell through hydrophilic pores and a pH-dependent active transport pathway in the inner cytoplasmic membrane Kircik (2010). Doxycycline is the preferred treatment for cats suspected of acute upper respiratory infections Lappin et al. (2017).
Table 3
|
Table 3 Multidrug-Resistant Isolates of Klebsiella Pneumoniae from Cats |
||
|
Total
Antibiotic |
Total
Resistance Isolates |
Percentage
(%) |
|
1 |
3 |
42.85% |
|
2 |
3 |
42.85% |
|
3 |
1 |
14.28% |
|
4 |
- |
- |
|
5 |
- |
- |
According to Table 3, 14.28% of the isolates are classed as multidrug-resistant (MDR) among the five antibiotics. The isolates were resistant to aminoglycosides, Extended spectrum cephalosporins or broad-spectrum cephalosporins (3rd and 4th generation cephalosporins), and fluoroquinolones. Isolates with multidrug resistance are resistant to at least three antibiotic groups. MDR-classified bacterial isolates will display multiple resistance profiles because, by definition, the organism must be resistant to at least three antimicrobial groups, even if there is only one resistant result for each group Magiorakos et al. (2012). According to Table 3, the other six isolates cannot be categorized as MDR since they are only resistant to one or two types of antibiotics. MDR can be produced in bacteria through one of two mechanisms. The first mechanism occurs when bacteria accumulate many drug-resistance-encoding genes within a single cell. Gene accumulation typically occurs on resistance (R) plasmids. The second step happens when the expression of genes producing efflux pumps for many pharmaceuticals is elevated, resulting in the rejection of numerous drugs Nikaido (2009).
Various percentages of Klebsiella pneumoniae isolates were resistant to four of the five studied antibiotics, namely cefotaxime, amoxicillin, gentamicin, and enrofloxacin. Antibiotics containing cefotaxime had the highest proportion, whereas the other four antibiotics had rates below 50%. Cefotaxime is less effective for treating Klebsiella pneumoniae bacterial infections, similar with the findings of earlier research demonstrating that Klebsiella pneumoniae bacteria are resistant to third generation cephalosporins Harada et al. (2016), Lee et al. (2021). Three isolates were amoxicillin-resistant, three were amoxicillin-sensitive, and one was intermediate. Numerous isolates are resistant to the antibiotic amoxicillin; therefore, its application must be evaluated. Only a small percentage of bacteria were resistant to gentamicin and enrofloxacin, indicating that they are still sensitive and effective in suppressing bacterial growth. The absence of resistant Klebsiella pneumoniae bacterial isolates in doxycycline test results indicated that the antibiotic remained effective for treating Klebsiella pneumoniae bacterial infections.
To obtain successful therapy, reduce patient safety risks,
and avoid the propagation of resistance, consideration must be given to the use
of antibiotics in the treatment of bacterial illnesses. One method for reducing
the spread of bacterial resistance to antibiotics is cautious use of
antibiotics. The sensible use of antibiotics is the use of antibiotics with a
narrow spectrum, in strict indications, and with the correct doses, intervals,
and administration duration. This usage recommendation features antibiotic
consumption restrictions and a modest reduction. The choice of antibiotic must
be based on knowledge of the spectrum of bacteria that cause infection and the
sensitivity pattern of bacteria to antibiotics, as well as the results of
microbiological studies of bacteria that cause infection, and it must be safe.
Additionally, the use of combination drugs or the administration of more than
one type of antibiotic can be used to slow the development of bacterial
resistance and reduce its risk.
4. CONCLUSION
Klebsiella pneumoniae bacteria isolated and identified from oropharyngeal swabs of hospitalized cats in Depok, West Java Indonesia, have acquired resistance to amoxicillin and cefotaxime. Klebsiella pneumoniae continues to be susceptible to the antibiotic’s gentamicin, enrofloxacin, and doxycycline.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
We appreciate the financing support from the Ministry of Research, Technology, and Higher Education, as well as the assistance provided by the Division of Medical Microbiology Research Laboratory workers during the research.
REFERENCES
Adler, K., Radeloff, I., Stephan, B., Greife, H., and Hellmann, K. (2007). Bakteriologischer Und Virologischer Status Bei Katzen Mit Erkrankungen Der Oberen Atemwege (Katzenschnupfenkomplex) [Bacteriological And Virological Status in Upper Respiratory Tract Infections of Cats (Cat Common Cold Complex)]. Berliner Und Münchener Tierärztliche Wochenschrift, 120(3–4), 120–125.
Balouiri, M., Sadiki, M., and Ibnsouda, S. K. (2016). Methods For in Vitro Evaluating Antimicrobial Activity : A Review. Journal of Pharmaceutical Analysis, 6(2), 71–79. https://doi.org/10.1016/j.jpha.2015.11.005.
Bart,
M., Guscetti, F., Zurbriggen, A., Pospischil, A., and Schiller, I. (2000).
Feline Infectious Pneumonia: A Short Literature Review and a Retrospective
Immunohistological Study on The Involvement of Chlamydia Spp. and Distemper
Virus. Veterinary Journal. London, England, 159(3), 220–230. https://doi.org/10.1053/tvjl.1999.0451.
Bouza, E., and Cercenado, E. (2002). Klebsiella and Enterobacter : Antibiotic Resistance and Treatment Implications. Seminars in Respiratory Infections, 17(3), 215–230.
Brisse, S., and Duijkeren, E. V. (2005). Identification and
Antimicrobial Susceptibility of 100 Klebsiella Animal Clinical Isolates. Veterinary
Microbiology, 105(3–4), 307–312. https://doi.org/10.1016/j.vetmic.2004.11.010.
Cappuccino, J. G. N., and Sherman. (2014). Microbiology A Laboratory Manual (10th). Pearson. [CLSI] Clinical and Laboratory Standards Institute. (2021). Performance Standards for Antimicrobial Susceptibility Testing, CLSI Supplement, M100. Ed Ke-28. Clinical and Laboratory Standards Institute.
Cowan, S. T., and Steel, S. (1993). Cowan and Steel’s Manual for the Identification of Medical Bacteria. Cambridge University Press. https://doi.org/10.1017/CBO9780511527104.
Epstein, S. E., Mellema, M. S., and Hopper, K. (2010). Airway
Microbial Culture and Susceptibility Patterns in Dogs and Cats With Respiratory
Disease of Varying Severity. Journal of Veterinary Emergency and Critical Care.
San Antonio, TX, 20(6), 587–594. https://doi.org/10.1111/j.1476-4431.2010.00587.x.
Foster, S. F., Martin, P., Allan, G. S., Barrs, V. R., And Malik, R. (2004). Lower Respiratory Tract Infections in Cats : 21 Cases (1995–2000). Journal of Feline Medicine and Surgery, 6(3), 167–180. https://doi.org/10.1016/j.jfms.2003.11.006.
Hakvoort,
H., Bovenkamp, E., Greenwood-Quaintance, K. E., Schmidt-Malan, S. M., Mandrekar,
J. N., Schuetz, A. N., and Patel, R. (2020). Imipenem-Relebactam
Susceptibility Testing of Gram-Negative Bacilli by Agar Dilution, Disk
Diffusion, and Gradient Strip Methods Compared With Broth Microdilution. Journal
of Clinical Microbiology, 58(10), E00695-20. https://doi.org/10.1128/JCM.00695-20.
Harada,
K., Shimizu, T., Mukai, Y., Kuwajima, K., Sato, T., Usui, M., Tamura, Y., Kimura,
Y., Miyamoto, T., Tsuyuki, Y., Ohki, A., and Kataoka, Y. (2016). Phenotypic
and Molecular Characterization of Antimicrobial Resistance In Klebsiella Spp. Isolates
from Companion Animals in Japan: Clonal Dissemination of Multidrug-Resistant
Extended-Spectrum Β-Lactamase-Producing Klebsiella Pneumoniae. Frontiers in
Microbiology, 7, 1021.
https://doi.org/10.3389/fmicb.2016.01021.
Herring, J. M. (2016). A Novel Placement Technique for Nasogastric and Nasoesophageal Tubes. Journal of Veterinary Emergency And Critical Care. San Antonio, TX, 26(4), 593–597. https://doi.org/10.1111/vec.12474.
Kircik, L. H. (2010). Doxycycline and Minocycline for The Management of Acne: A Review of Efficacy and Safety With Emphasis on Clinical Implications. Journal of Drugs in Dermatology: JDD, 9(11), 1407–1411.
Lappin,
M. R., Blondeau, J., Boothe, D., Breitschwerdt, E. B., Guardabassi, L., Lloyd, D.
H., Papich, M. G., Rankin, S. C., Sykes, J. E., Turnidge, J., and Weese, J. S.
(2017). Antimicrobial Use Guidelines for Treatment Of Respiratory Tract
Disease in Dogs and Cats: Antimicrobial Guidelines Working Group of the International
Society for Companion Animal Infectious Diseases. Journal of Veterinary
Internal Medicine, 31(2), 279–294. https://doi.org/10.1111/jvim.14627.
Lee,
D., Oh, J. Y., Sum, S., and Park, H. M. (2021). Prevalence and
Antimicrobial Resistance of Klebsiella Species Isolated from Clinically Ill
Companion Animals. Journal of Veterinary Science, 22(2), E17. https://doi.org/10.4142/jvs.2021.22.e17.
Li, Z. J., Zhang, H. Y., Ren, L. L., Lu, Q. B., Ren, X., Zhang, C. H., Wang,
Y. F., Lin, S. H., Zhang, X. A., Li, J., Zhao, S. W., Yi, Z. G., Chen, X., Yang,
Z. S., Meng, L., Wang, X. H., Liu, Y. L., Wang, X., Cui, A. L. (2021). Etiological
and Epidemiological Features of Acute Respiratory Infections In China. Nature
Communications, 12(1), 5026.
https://doi.org/10.1038/s41467-021-25120-6.
Magiorakos, A. P., Srinivasan, A., Carey, R. B., Carmeli, Y., Falagas,
M. E., Giske, C. G., Harbarth, S., Hindler, J. F., Kahlmeter, G., Olsson-Liljequist,
B., Paterson, D. L., Rice, L. B., Stelling, J., Struelens, M. J., Vatopoulos, A.,
Weber, J. T., And Monnet, D. L. (2012). Multidrug-Resistant, Extensively
Drug-Resistant and Pandrug-Resistant Bacteria : An International Expert
Proposal for Interim Standard Definitions for Acquired Resistance. Clinical
Microbiology and Infection, 18(3), 268–281. https://doi.org/10.1111/j.1469-0691.2011.03570.x.
Nikaido, H. (2009). Multidrug Resistance in Bacteria. Annual Review of Biochemistry, 78, 119–146. https://doi.org/10.1146/annurev.biochem.78.082907.145923.
National Nosocomial Infections Surveillance System [NNIS] (2004).
National Nosocomial Infections Surveillance (NNIS) System Report, Data Summary
from January 1992 Through June 2004, Issued October 2004. American Journal of
Infection Control, 32(8), 470–485. https://doi.org/10.1016/s0196655304005425.
Podschun,
R., and Ullmann, U. (1998). Klebsiella Spp. as Nosocomial Pathogens: Epidemiology,
Taxonomy, Typing Methods, and Pathogenicity Factors. Clinical Microbiology
Reviews, 11(4), 589–603.
https://doi.org/10.1128/CMR.11.4.589.
Quinn, P. J., Markey, B. K., Leonard, F. C., Fitzpatrick, E. S., Fanning, S., And Hartigan, P. J. (2011). Veterinary Microbiology and Microbial Disease. Blackwell Publishing. https://doi.org/10.1016/j.vetmic.2012.06.004.
Schulz,
B. S., Wolf, G., and Hartmann, K. (2006). Bacteriological and Antibiotic
Sensitivity Test Results in 271 Cats With Respiratory Tract Infections. Veterinary
Record, 158(8), 269–270.
https://doi.org/10.1136/vr.158.8.269.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2022. All Rights Reserved.