
A RETROSPECTIVE AND PROSPECTIVE STUDY FOR ADVERSE DRUG REACTION OF CANCER CHEMOTHERAPY IN BREAST CANCER
Saket Saini 1![]()
![]() ,
Alishan Zia 2
,
Alishan Zia 2![]() , Shaily Tyagi 3
, Shaily Tyagi 3![]() , Himani Nautiyal
4
, Himani Nautiyal
4![]()
1 Siddhartha Institute of Pharmacy, Dehradun, Uttarakhand, India
2 Delhi Institute of Pharmaceutical
Sciences and Research, Delhi, India
3 Assistant professor in Quantum
university, Roorkee, Uttarakhand, India
4 Associate professor in Siddhartha Institute, Dehradun, Uttarakhand, India
|
|
ABSTRACT |
||
|
Cancer is the major health problem worldwide, according to WHO in 2018 cancer is responsible for an estimated 9.6 mill death out of 18.1 million new cases and globally 6 deaths occur due to cancer. Cancer is the abnormal, unwanted growth of cells, when abnormal cells divide in an uncontrolled way within the body and destroy normal tissue that can lead to death. Normal body cell grows, divide, and die in an orderly way or cancer cells are different because they do not die, grow continuously, and divide in orderly way. There are more than 200 types of cancer. Any agent that converts a body cell to develop abnormally can cause cancer. The cancer causative agents include- radiations, chemical or toxic compound exposure, lifestyle, hormones, and human genetics. Most tumors can be categorized as one of the three main groups: carcinomas, sarcomas and leukemia or lymphomas. Carcinomas, which associated around 90% of human cancer, are malignancies of epithelial cell. Sarcomas, which are uncommon in human, are solid tumor of connective tissue including muscle, bone, cartilage, and fibrous tissue. Leukemia and lymphomas, which represent around 8% of human malignancies, emerge from the blood forming cell and from cell of immune system. Carcinoma of breast is a malignant disease with variable outcomes. Breast carcinoma is second most common cause of death in females though it can also occur in men but are very uncommon. This type of cancer is malignant cell growth in breast. This study was done to compare and analyze adverse drug reactions of cancer chemotherapy in breast cancer in a rural hospital. Objectives included- a) To study adverse drug reactions of various chemotherapeutic agents used in patients suffering breast cancer b) To compare various side-effects as a result of different combination protocols to identify the drug protocol with least amounts of adverse drug reactions. However, an extremely significant difference was found between all of the comparison groups studied. |
|||
|
Received 01 November 2022 Accepted 02 December 2022 Published 15 December 2022 Corresponding Author Saket
Saini, shailytyagi664@gmail.com DOI10.29121/granthaalayah.v10.i11.2022.4922 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2022 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Breast Cancer, Carcinomas, Sarcomas,
Leukemia, Lymphomas, Women |
|||
1. INTRODUCTION
The incidence of breast cancer increasing day by day in India. Breast cancer is second most frequent cause of death among women. Breast cancer also occurs in men but it’s very far less common. In this type of condition, cancer may be present as a lump or nipple discharge Sharma et al. (2010) Most of the breast cancers are adenocarcinomas which arise from glandular tissue and there are about 30 different subtypes of adenocarcinoma.
Adenocarcinoma is a type of cancer affecting glands. In case of breast cancer adenocarcinoma starts from the glands of breast where milk produce.
2. TYPES OF BREAST CANCER
Adenocarcenoma is categorized in two parts –
1) Ductal carcinoma in situ
2) Lobular carcinoma in situ
These are the form of breast cancer. In Ductal carcinoma in situ abnormal cell are present but still contained in the milk duct. Lobular carcinoma in situ is found in the breast lobules but not yet surrounding tissue.
3. OTHER COMMON TYPES OF BREAST CANCER
· Inflammatory breast cancer
· Medullary breast cancer
· Paget disease of the breast
· Papillary breast carcinoma
· Invasive ductal carcinoma
1) Lobular carcinoma in situ This type of cancer develops in the milk glands (lobules) of the breast. Lobule is a gland that produces milk.
The term “In Situ” refers that the abnormal cells are present in the same place where they first formed or they have not spread. The lobules of the breast's exterior have not been affected by LCIS.
2) Ductal cancer in situ In DCIS the abnormal cells present inside the milk duct in the breast. Both LCIS & DCIS identified non breast cancer that has spread.
When aberrant cells within the milk duct or lobules burst and infiltrate the surrounding tissue, invasive breast cancer is the result. Cancer cells spread throughout the body from the breast to other areas via the systemic circulation.
The most common invasive breast cancer includes:
1) Invasive ductal carcinoma that begin in the milk duct
2) Invasive lobular carcinoma that begin in the lobules.
3) Breast cancer that is inflammatory results from cancer cells obstructing lymphatic arteries or channels in the skin above the breast. The breast seems enlarged, red, and inflamed; this is why it is called an inflammatory condition.
4) Paget disease in the breast This extremely uncommon type of breast cancer begins on the nipple and spreads to the area around the nipple. About 1-4.3% of all breast cancer cases were this form of malignancy. It is connected to underlying ductal carcinoma, either invasive or in situ.
4. ETIOLOGY AND RISK FACTOR
A risk factor is anything that increases the risk of breast cancer. The risk factor of breast cancer is unknown, many women who suffer breast cancer have no known factor like simple being women. The studies suggest that the main risk factor that is associated with an increase risk of breast cancer include:
1) Studies show that the main risk factor of breast cancer includes being women. Breast cancer develops in women more likely than men.
2) Age Breast cancer is increasing with age, most breast cancer is diagnosed after age 50 year.
3) Family History A women have higher risk if she has a first degree relative or multiple relative family member affect by breast cancer.
4) Drinking Alcohol Studies show that the women’s risk is increases with drinks more alcohol.
5) Radiation therapy If you received radiation therapy as a child or young, the risk of breast cancer is increases.
6) Hormonal factors the women who take hormone therapy (include estrogen and progesterone) during menopause can raise the risk for breast cancer.
7) Heredity Breast cancer development is a complex process involving mutational and genetic event. Gene wide association’s studies suggest that multiple mutations are present in breast cancer and more than 20 mutations identified, each of which impart a small increase in frequency of breast cancer. The most common mutation includes BRCA1and BRCA 2 genes that are associated with approximately 5%of breast cases.
8) Obesity The studies suggest that the dietary fat increase the risk of breast cancer. Intake high calories lead to weight gain and obesity. Obesity increases the risk of breast cancer because obese women may have an increased exposure of breast tissue to estrogen that results hyper estrogenic state. Mansfield (1993)
5. SIGN AND SYMPTOMS OF BREAST CANCER
The sign and symptoms of breast cancer are depending upon
the type of breast cancer. Different types of breast cancer produce varieties
of symptoms some of which may be common while others may be different. The most
common sign &symptoms of breast cancer may include: Ames
et al. (1995)
· The size shape or appearance of a breast is change.
· The most common sign of breast cancer is a lump or thickening in the breast that feel different from the surrounding tissue.
· Skin over the breast is change, such as dimpling.
· Nipple discharge
· Change in the breast color.
· Peeling or flaking of skin surrounding nipple.
· Redness, swelling, itching and rashes on the breast.
· Change the shape of nipple.
6. SCREENING
Screening can be done to detect breast cancer as soon as possible to provide better treatment. Many screening programs have been intended to detect small, early cancer. The breast cancer screening can be done with Mammography and other clinical examination like ultrasound and MRI. The mortality is decrease with mammography screening and a report of randomized control trial show the 31 % mortality is decrease. Women aged 40 or above required a routine screening programmed at every 1to 2 year, whether or not symptoms are clear. Berry et al. (2005)
7. TREATMENT
There are different treatments approaches of breast cancer, treatment depend upon the type and size of tumor. The main treatment of breast cancer is surgery, chemotherapy, radiotherapy, and hormone therapy. In surgery mostly modified redical mastectomy, simple mastectomy and quadrantectomy are done. Post operative chemotherapy is given after surgery in all cases. In radiation therapy a beam of energy is designed to kill breast cancer. Radiation therapy can be used before the surgery to shrink large tumor or after surgery to kill remaining breast cancer cell. Radiation therapy can be given surrounding the area where cancer is present. Cameron et al. (1994), Arruebo et al. (2011).
8. REVIEW OF LITERATURE
8.1. DOXORUBICIN
It is an antibiotic from Anthracycline family used in the treatment of cancer including breast, lungs, gastric, ovarian, thyroid, sarcoma, pediatric cancer etc.
1) Thorn et al. (2011): Doxorubicin inhibits topoisomerase II which is responsible for DNA synthesis. Doxorubicin inhibits macromolecular production by intercalating into DNA. The anticancer chemical used in Dox-based chemotherapy affects healthy tissue, causing toxicity and adverse effects, and does not only target breast cancer.
Cardiotoxicity is the primary hazardous consequence, and usage of the substance can result in the development of cardiomyopathy, which can progress to biventricular failure and even death. Acute toxicities of Doxorubicin include nausea, vomiting, myelosuppression, alopecia and mucositis. Myelosupression is the acute dose limiting toxicity wherein the WBC.
2) Zoli et al. (n.d.) reported that Doxorubicin and paclitaxel in combination show synergistic cytocidal effects. After the sequence of Dox-Pacl apoptosis was observed.
3) Luikart et al. 1984: A combination of AVM (Doxorubicin, Vinblastin & Mitomycin) the major toxicity was myelosuppression. In some cases leucopenia, thrombocytopenia and anemia was found. One patient receives CMF (cyclophosphamide, methotrexate & 5-flurouracil) developed acute myelogenous leukemia after starting AVM.
4) Zhao et al. 2017: The most important side effects of pegylated liposomal doxorubicin in adjuvant chemotherapy for breast cancer were skin toxicity, stomatitis and hand-foot syndrome.
5) Song et al. (2011): Anthracyclines based regimens have been the backbone in adjuvant chemotherapy. These are cardiotoxic and makes serious and irreversible damage on heart. Studies show that congestive heart failure is the main risk of higher dose of doxorubicin.
8.2. CYCLOPHOSPHAMIDE
Cyclophosphamide is nitrogen mustard alkylating agent used in different type of cancer including breast cancer. It inhibits DNA, RNA, and protein synthesis by acting on cross linking purine base in DNA. Toxic effects of cyclophosphamide include bone marrow suppression, cardiotoxicity and hemorrhagic sistitis. Cardiotoxicity may occur when very high dose are given. Common side effects are nausea and vomiting which is well controlled by antiemetic drugs.
1) Song et al. (2011): The main toxic effects of TC (Docetaxel &cyclophosphamide) regimen were neutropenia and common non hematological toxicity developed in patients was hair loss, fatigue, nausea, and vomiting. Other side effects include constipation, peripheral neuropathy, and hyperglycaemia in some cases.
2) North R. J. 1982: Cyclophosphamide is used in breast and ovarian cancer at much lower doses as a single agent or as adjuvant treatment for treat lymphomas. The effectiveness of low dose of cyclophosphamide is essentially because of its capacity to promote antitumor immunity by selective depletion of regulatory T cell and enhancement of effectors T cell functions.
3) Castel et al. (2013): Congestive Heart failure is the most serious cardiac complication due to cyclophosphamide at high dose. Other common side effects include hypotension, hypertension, arrhythmias and conduction disturbances, pericarditis, and thromboembolic complications.
4) Karisson Y. A. et al. (1998): In a study report of 128 Patients in the treatment of advanced breast cancer with FEC (5-flurouracil, epirubicin and cyclophosphamide) regimen, no cardiac failure or toxic death were recorded. The common toxicities alopecia and nausea were observed.
9. METHODOLOGY
Cases undergoing chemotherapy for breast cancer were included and patients had to be above 18 years of age.
Inclusion criteria were-
1) Adjuvant chemotherapy
2) Palliative chemotherapy in advance cases
Exclusion criteria-
1) Pregnant women
2) Neonates
10. DOSES USED IN THIS STUDY
10.1. IN THIS STUDY DIFFERENT TYPE’S COMBINATION CHEMOTHERAPY PROTOCOL ARE USED
· CAF: Cyclophosphamide 600-1000mg/m2+ Doxorubicin 60-75mg/m2+ 5-Flurouracil 500mg/m2
· FMC: 5-Flurouracil 500mg/m2 + Methotrexate 40-80mg/m2 + Cyclophosphamide 600- 1000mg/m2
· DC: Docetaxel 60-100mg/m2 + carboplatin 250mg/m2 PC: Paclitaxel 135-225mg/m2 + cisplatin 40-120mg/m2. Shewach and Kuchta (2009)
10.2. CALCULATION OF BODY SURFACE AREA
Direct measurements is difficult so various formulae is used to calculate the Body Surface area (BSA) which is used in many measurements in medicine including the calculation of drug dosages and the amount of fluid to be administered IV. Some formulae are listed below:
1) Du Bois formula: BSA = 0.007184 X W0.425 X H0.725
2) Mosteller formula: BSA = 0.016667 X W0.5 H0.5
3) Haycock formula: BSA = 0.0235 X W0.5378 X H0.3964
4) Gehan and George formula: BSA = 0.0235 X W0.51456 X H0.42246
5) Boyd formula: BSA = 0.03330 X W(0.6157-0.0188 X LOG 10(W) X H0.3
6) Fujimoto formula: BSA = 0.008883 X W 0.444 X H 0.663
7) Takahira formula: BSA = 0.007241 X W 0.425 X H 0.725
8) Shlich formula: BSA = 0.000975482 X W 0.46 X H 1.08 (Women) BSA = 0.000579479 X W 0.38 X H 1.24 (Men)
NOTE: In this study Du Bois formula has been used.
11. DOSE CALCULATION
Drug doses are calculated according to the patient’s body surface area and occasionally, by height. For this study, accurate weight was used for great consistency of drug dosing.
Side effects observed in this study
1) Nausea
2) Vomiting
3) Diarrhea
4) Alopecia
5) Weight loss
6) Stomatitis
7) Constipation
8) Palpitation
9) Cardiotoxicity
10) Neutropenia
11) Loss of appetite
12) Fever
13) Skin rashes
14) Thrombocytopenia
15) Shock
16) Nail blackening
17) Leukopenia
18) Neuropathy
19) Inflammation
20) Anemia
21) Haematuria
22) Hyperacidity
23) Depression
24) Mood swing
Table 1
|
Table 1 Table Showing Combination Chemotherapy Protocol Used in this Study |
|||
|
S. No |
Protocol used |
NO of patients |
% of Patients |
|
1 |
CMF |
10 |
25 |
|
2 |
FAC |
10 |
25 |
|
3 |
DC |
10 |
25 |
|
4 |
PC |
10 |
25 |
12. RESULT AND OBSERVATIONS
Table 2
|
Table 2 Table Showing Gender Distribution |
|||
|
S. No |
Sex |
Number of Patient |
Percentage |
|
1 |
Male |
0 |
0% |
|
2 |
Female |
40 |
100% |
Table 3
|
Table 3 Table Showing Chances of Disease Occurrence on the Basis of Age |
|||
|
S. No |
Age in Year |
No. of Patient |
Percentage |
|
1 |
20 – 25 |
0 |
0% |
|
2 |
26 – 30 |
1 |
2.5% |
|
3 |
31 – 35 |
2 |
5% |
|
4 |
36 – 40 |
2 |
5% |
|
5 |
41 – 45 |
9 |
22.5% |
|
6 |
46 – 50 |
10 |
25% |
|
7 |
51 – 55 |
7 |
17.5% |
|
8 |
56 – 60 |
5 |
12.5% |
|
9 |
61 – 65 |
3 |
7.5% |
|
10 |
66 – 70 |
1 |
2.5% |
|
11 |
70 above |
0 |
0% |
Table 4
|
Table 4 Side Effects Seen in Cyclophosphamide, Methotrexate & 5-FU Protocol |
|||
|
S. No |
Side effects |
No of Patient |
Percentage |
|
1 |
Nausea |
10 |
100% |
|
2 |
Vomiting |
10 |
100% |
|
3 |
Alopecia |
9 |
90% |
|
4 |
Loss of appetite |
8 |
80% |
|
5 |
Weight loss |
6 |
60% |
|
6 |
Stomatitis |
3 |
30% |
|
7 |
Constipation |
1 |
10% |
|
8 |
Palpitation |
1 |
10% |
|
9 |
Anorexia |
3 |
30% |
|
10 |
Neutropenia |
4 |
40% |
|
11 |
Diarrhea |
7 |
70% |
|
12 |
Mood swing |
2 |
20% |
|
13 |
Skin rashes |
1 |
10% |
|
14 |
Fever |
3 |
30% |
|
15 |
Leukopenia |
3 |
30% |
|
16 |
Thrombocytopenia |
1 |
10% |
|
17 |
Mettalic taste |
3 |
30% |
|
18 |
Insomnia |
2 |
20% |
|
19 |
Weakness |
6 |
60% |
|
20 |
Neuropathy |
1 |
10% |
|
21 |
Inflammation |
2 |
20% |
|
22 |
Anemia |
3 |
30% |
|
23 |
Haematuria |
1 |
10% |
|
24 |
Shock |
2 |
20% |
|
25 |
Nail blackening |
1 |
10% |
|
26 |
Hyperacidity |
5 |
50% |
Table 5
|
Table 5 Side Effects Seen In 5-FU, Doxorubicin and Cyclophosphamide Protocol |
|||
|
S. No |
Side effects |
No of Patient |
Percentage |
|
1 |
Nausea |
10 |
100% |
|
2 |
Vomiting |
10 |
100% |
|
3 |
Diarrhea |
6 |
60% |
|
4 |
Alopecia |
8 |
80% |
|
5 |
Weight loss |
7 |
50% |
|
6 |
Stomatitis |
4 |
60% |
|
7 |
Shock |
1 |
60% |
|
8 |
Palpitation |
2 |
40% |
|
9 |
Cardiotoxicity |
1 |
10% |
|
10 |
Thrombocytopenia |
3 |
20% |
|
11 |
Loss of appetite |
6 |
60% |
|
12 |
Fever |
4 |
40% |
|
13 |
Anorexia |
2 |
60% |
|
14 |
Netropenia |
5 |
60% |
|
15 |
Leukopenia |
2 |
30% |
|
16 |
Skin rashes |
3 |
50% |
|
17 |
Mettalic taste |
3 |
55% |
|
18 |
Insomnia |
3 |
45% |
|
19 |
Weakness |
5 |
45% |
|
20 |
Neuropathy |
2 |
7.5% |
|
21 |
Inflammation |
1 |
5% |
|
22 |
Anemia |
2 |
10% |
|
23 |
Haematuria |
2 |
7.5% |
|
24 |
Hyperacidity |
6 |
50% |
|
25 |
Depression |
2 |
30% |
|
26 |
Mood swing |
1 |
20% |
|
27 |
Chest pain |
1 |
7.5% |
Table 6
|
Table 6 Side Effects in Docetaxel and Carboplatin Protocol |
|||
|
S. No |
Side effects |
No of Patient |
Percentage |
|
1 |
Nausea |
10 |
100% |
|
2 |
Vomiting |
10 |
100% |
|
3 |
Diarrhea |
6 |
60% |
|
4 |
Alopecia |
3 |
30% |
|
5 |
Weight loss |
5 |
50% |
|
6 |
Stomatitis |
3 |
30% |
|
7 |
Constipation |
1 |
10% |
|
8 |
Palpitation |
1 |
10% |
|
9 |
Cardiotoxicty |
2 |
20% |
|
10 |
Shock |
1 |
20% |
|
11 |
Loss of appetite |
5 |
50% |
|
12 |
Fever |
4 |
40% |
|
13 |
Skin rashes |
2 |
20% |
|
14 |
Neutropenia |
3 |
30% |
|
15 |
Leukopenia |
1 |
10% |
|
16 |
Thrombocytopenia |
1 |
10% |
|
17 |
Mettalic taste |
2 |
20% |
|
18 |
Insomnia |
2 |
20% |
|
19 |
Weakness |
7 |
70% |
|
20 |
Neuropathy |
1 |
10% |
|
21 |
Inflammation |
1 |
10% |
|
22 |
Anemia |
1 |
10% |
|
23 |
Haematuria |
1 |
10% |
|
24 |
Hyperacidity |
4 |
40% |
|
25 |
Depression |
1 |
10% |
|
26 |
Anorexia |
1 |
10% |
Table 7
|
Table 7 Side Effects Seen in Paclitaxel and Cisplatin Protocol |
|||
|
S. No |
Side effects |
No of Patient |
Percentage |
|
1 |
Nausea |
10 |
100% |
|
2 |
Vomiting |
10 |
100% |
|
3 |
Diarrhea |
5 |
50% |
|
4 |
Alopecia |
4 |
40% |
|
5 |
Weight loss |
5 |
50% |
|
6 |
Stomatitis |
2 |
20% |
|
7 |
Weakness |
5 |
50% |
|
8 |
Palpitation |
2 |
20% |
|
9 |
Cardiotoxicty |
1 |
10% |
|
10 |
Leucopenia |
2 |
20% |
|
11 |
Loss of appetite |
6 |
60% |
|
12 |
Fever |
2 |
20% |
|
13 |
Skin rashes |
1 |
10% |
|
14 |
Thrombocytopenia |
2 |
20% |
|
15 |
Hyperacidity |
5 |
50% |
|
16 |
Mood swing |
1 |
10% |
|
17 |
Mettalic taste |
3 |
30% |
|
18 |
Insomnia |
1 |
1% |
|
19 |
Anorexia |
1 |
1% |
|
20 |
Neuropathy |
3 |
3% |
|
21 |
Inflammation |
2 |
2% |
|
22 |
Anemia |
2 |
20% |
|
23 |
Neutropenia |
4 |
40% |
|
24 |
Nail blacknening |
1 |
10% |
Table 8
|
Table 8 According to Side Effects Distribution |
|||
|
S. No |
Side effects |
No. of Patient |
Percentage |
|
1 |
Nausea |
40 |
100% |
|
2 |
Vomiting |
40 |
100% |
|
3 |
Diarrhea |
24 |
60% |
|
4 |
Alopecia |
24 |
60% |
|
5 |
Weight loss |
23 |
57.5% |
|
6 |
Stomatitis |
12 |
30% |
|
7 |
Constipation |
2 |
5% |
|
8 |
Palpitation |
6 |
15% |
|
9 |
Cardiotoxicty |
4 |
10% |
|
10 |
Neutropenia |
16 |
40% |
|
11 |
Loss of appetite |
25 |
62.5% |
|
12 |
Fever |
13 |
32.5% |
|
13 |
Skin rashes |
6 |
15% |
|
14 |
Thrombocytopenia |
7 |
17.5% |
|
15 |
Shock |
4 |
10% |
|
16 |
Nail blackening |
2 |
5% |
|
17 |
Leukopenia |
8 |
20% |
|
18 |
Mettalic taste |
11 |
27.5% |
|
19 |
Insomnia |
8 |
20% |
|
20 |
Weakness |
23 |
57.5% |
|
21 |
Neuropathy |
7 |
17.5% |
|
22 |
Inflammation |
6 |
15% |
|
23 |
Anemia |
8 |
20% |
|
24 |
Haematuria |
4 |
10% |
|
25 |
Hyperacidity |
20 |
50% |
|
26 |
Depression |
3 |
7.5% |
|
27 |
Mood swing |
4 |
10% |
|
28 |
Chest pain |
1 |
2.5% |
|
29 |
Anorexia |
7 |
17.5% |
Table 9
|
Table 9 Percentage of Patients Showing ADR-Vomiting |
|||
|
S. No |
Drug regimen |
No of Patient |
Percentage |
|
1 |
CMF |
10 |
100% |
|
2 |
FAC |
10 |
100% |
|
3 |
DC |
10 |
100% |
|
4 |
PC |
10 |
100% |
Graph 1
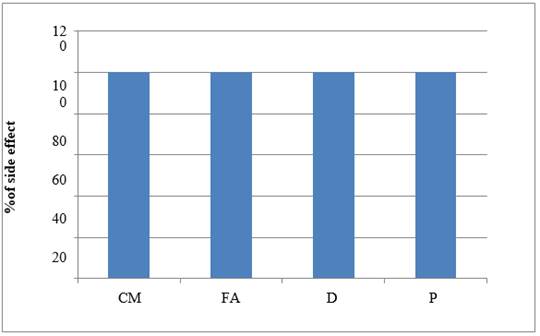
|
Graph 1 Graph Showing ADR –Vomiting |
Graph 2
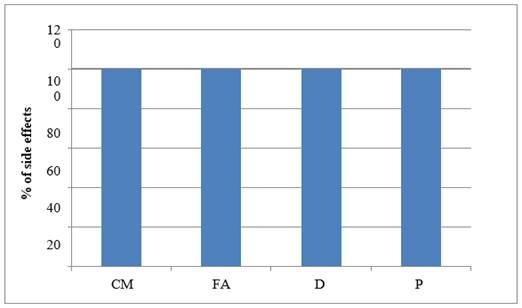
|
Graph 2 Graph Showing ADR –Nausea |
Graph 3
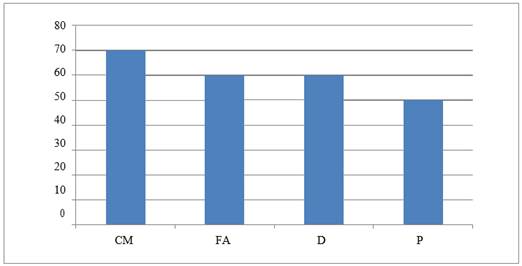
|
Graph 3 Graph Showing ADR – Diarrhea |
Graph
4
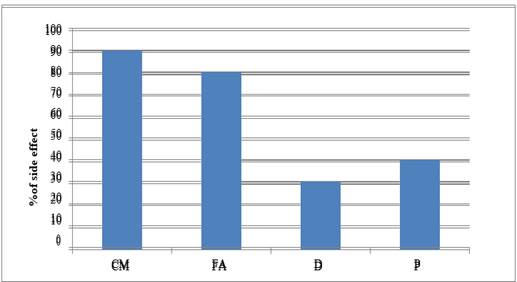
|
Graph 4 Graph Showing ADR –Alopecia |
Graph 5
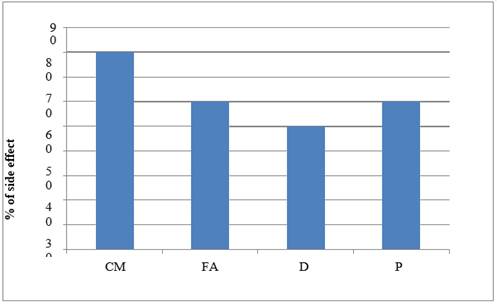
|
Graph 5 Graph Showing ADR –Loss of Appetite |
Graph 6
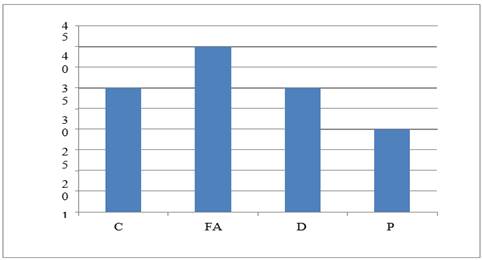
|
Graph 6 Graph Showing ADR –Stomatitis |
Graph 7
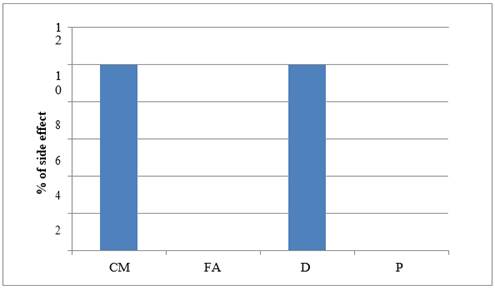
|
Graph 7 Graph Showing ADR –Constipation |
Graph 8
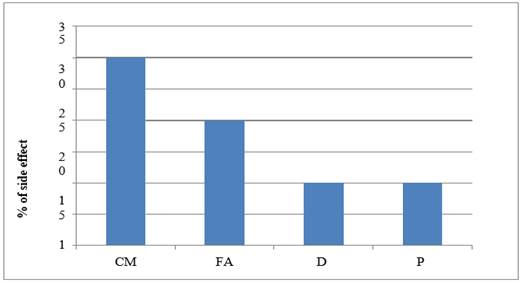
|
Graph 8 Graph Showing ADR –Anorexia |
Table 10
|
Table 10 Table Demonstrating Studied Adverse Drug Reactions with Statistical Significance |
|
|
Adverse drug
reaction |
P
value |
|
Vomiting |
<0.0001 |
|
Nausea |
<0.0001 |
|
Diarrhea |
<0.0001 |
|
Alopecia |
<0.0001 |
|
Loss of appetite |
<0.0001 |
|
Stomatitis |
<0.0001 |
|
Constipation |
<0.0001 |
|
Weight loss |
<0.0001 |
|
Palpitation |
<0.0001 |
|
Skin rashes |
<0.0001 |
|
Neutropenia |
<0.0001 |
|
Cardiotoxicity |
<0.0001 |
|
Fever |
<0.0001 |
|
Thrombocytopenia |
<0.0001 |
|
Leucopenia |
<0.0001 |
|
Metallic taste |
<0.0001 |
|
Insomnia |
<0.0001 |
|
Weakness |
<0.0001 |
|
Shock |
<0.0001 |
|
Nail blackening |
<0.0001 |
|
Neuropathy |
<0.0001 |
|
Inflammation at injection site |
<0.0001 |
|
Anemia |
<0.0001 |
|
Hematuria |
<0.0001 |
|
Hyperacidity |
<0.0001 |
|
Depression |
<0.0001 |
|
Chest pain |
<0.0001 |
|
Mood swings |
<0.0001 |
|
Anorexia |
<0.0001 |
13. STATISTICAL ANALYSIS
Unpaired T test was employed as statistical tool and significance was determined based on P values i.e., P<0.5 – significant; <0.001- highly significant and <0.0001- extremely significant.
14. CONCLUSION
Highest case of breast cancer was found in 41 to 45 year of age group. Nausea and vomiting resulted from all the studied drug protocols used for treating breast cancer. The cyclophosphamide- methotrexate and 5- fluorouracil drugs protocol showed maximum case of alopecia (in 90% patients) followed in decreasing order as follows- appetite loss (80%), diarrhea (70%), weight loss (60%), hyperacidity (50%), neutropenia (40%), anemia, leucopenia, stomatitis, anorexia, and metallic taste (30%), skin rashes, nail blackening (10%).
The 5- fluorouracil- doxorubicin and cyclophosphamide protocol presented with alopecia (80%) followed by- diarrhea, stomatitis, shock, appetite loss, anorexia and neutropenia (60%), hyperacidity, weight loss and skin rashes (50%), insomnia and weakness (45%), palpitation (40%), metallic taste and depression (30%), mood swings (20%). Chest pain, hematuria and neuropathy (7.5% cases). In Docetaxel and carboplatin protocol, diarrhea was most common adverse effect (60%) which was followed by – weight loss and loss of appetite (50%), fever (40%), stomatitis, alopecia, and neutropenia in 30% cases. In the Paclitaxel and cisplatin protocol, loss of appetite was seen in 60% cases followed by diarrhea, weakness and hyperacidity in 50% and alopecia and neutropenia in 40% cases. All study group showed extremely significant differences (P<0.0001) between the adverse drug reaction studied.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
Ames, B. N., Gold, L. S. and Willett, W. C. (1995). The
Cause and Prevention of Cancer. the Proceedings of the National Academy of
Sciences, 92(12), 5258-5265.
https://doi.org/10.1073/pnas.92.12.5258.
Brown, A., Kumar, S. and Tchounwou, P. B. (2019). Cisplatin Based Chemotherapy of Human Cancers. Journal of Cancer Science and Therapy 11(4).
Arruebo, M., Vilaboa, N., and Saez-Gutierrez, B. (2011). Assessment of the Evolution of Cancer Treatment Therapies. Cancers 3(3), 3279-3330. https://doi.org/10.3390/cancers3033279.
Berry, D. A., Cronin, K. A., and Plevritis, S. K. (2005). Effect of Screening and Adjuvant Therapy on Mortality From Breast Cancer. The New England Journal of Medicine, 353, 1784-1792. https://doi.org/10.1056/NEJMoa050518.
Berry, D., Cronin, K., Zelen, M., (2005).
Effect of Screening and Adjuvant Therapy on Mortality from Breast Cancer. The
New England Journal of Medicine, 353, 1784-1792. https://doi.org/10.1056/NEJMoa050518.
Boulikas,
T., Vougiouka, M. (2004). Recent Clinical Trials Using Cisplatin,
Carboplatin and Their Combination Chemotherapy Drugs (Review). Oncology
Reports, 11, 559-95.
https://doi.org/10.3892/or.11.3.559.
Brahmachari, B., Hazra, A. and Mazumdar, A. (2011). Adverse Drug Reaction Profile of Nanoparticle Versus Comventional Formulation of Paclitaxel : An Observational Study. Indian Journal of Pharmacology, 126-130. https://doi.org/10.4103/0253-7613.77341.
Cameron,
D. A., Gabra, H. and Leonard, R. C. (1994). "Continuous
5-Flurouracil in the Treatment of Breast Cancer". British Journal of
Cancer, 120-124.
https://doi.org/10.1038/bjc.1994.259.
Castel, M., Despas,F. Modesto, A., Gales, C., Honton, B., Galinier, M., Senard, J. M., Pathak, A.(2013). Cardiotoxicity of Chemotherapies. Presse Med. 26-39. https://doi.org/10.1016/j.lpm.2012.04.014.
Chan, A., Su, C., De Boer, R.H., and Gajdatsy, A. (2013). Prevalence of Excessive Tearing In Women With Early Breast Cancer Receiving Adjuvant Docetaxel-Based Chemotherapy. Journal of Clinical Oncology, 31(17), 2123-2127. https://doi.org/10.1200/JCO.2012.45.6574.
Coley, H.M. (2008). Mechanisms and Strategies to Overcome Chemotherapy Resistance in Metastatic Breast Cancer. Cancer Treatment Reviews , 34, 378-390. https://doi.org/10.1016/j.ctrv.2008.01.007.
Crown, J., Hakes,T. and Reichman, B.(1993). Carboplatin and Etoposide In Metaststic Breast Cancer. Cancer 74, 1254-1257.
Desantis, C., Siegel, R., Bandi, P., and Jemal, A. (2011). A Breast Cancer Statistics, 2011. CA : A Cancer Journal For Clinicians, 61, 408-418. https://doi.org/10.3322/caac.20134.
Doroshow, J.H., Leong, L., Margolin, K. (1989). Refractory Metastatic Breast Cancer: Salvage Therapy with Fluorouraciland High-Dose Continuous Infusion Leucovorin Calcium. Journal of Clinical Oncology, 439-744. https://doi.org/10.1200/JCO.1989.7.4.439.
Ejlersten, B., Mouridsen, H.T., Jensen, M.B. (2008). Adjuvant Cyclophosphamide, Methotrexate and Fluorouracil in Premenopausal Patients with Node Positive Breast Cancer : Indirect Comparison of Dose and Schedule in DBCG Trials 77, 82 and 89. Acta Oncologica 47(4), 662-71. https://doi.org/10.1080/02841860801989761.
Emadi, A., Jones, R.J. and Brodsky, R.A. (2009). "Cyclophosphamide and Cancer : Golden Anniversary" Nature Review Clinical Oncology, 6, 638-647. https://doi.org/10.1038/nrclinonc.2009.146.
Etienne, M-C., Guillot, T., Milano, G. (1996).
Critical Factors Optimizing the 5-fluorouracil-Folinic Acid Association in
Cancer Chemo-Therapy. Acta Oncologica, 7, 283-289. https://doi.org/10.1093/oxfordjournals.annonc.a010573.
Ferlay, J., Colombet, M., Soerjomataram, I., Matthers, C., Parkin, D. M., Pineros, M., Znaor, A. and Bray, F. (2018). Estimating the Global Cancer Incidence and Mortality in 2018 : GLOBOCAN Sources and Methods International Journal of Cancer 1941-1953. https://doi.org/10.1002/ijc.31937.
Florea, A. M., and Busselberg, D. (2011).
Cisplatin as an Antitumor Drug: Cellular Mechanisms of Activity, Drug Resistance
and Induced Side Effects. Cancers 3(1), 1351- 1371. https://doi.org/10.3390/cancers3011351.
Groopman, J.E., and Itri, L.M. (1999). Chemotherapy-Induced Anemia in Adults: Incidence and Treatment. Journal of the National Cancer Institute 91(19), 1616-34. https://doi.org/10.1093/jnci/91.19.1616.
Gabra, H., Cameron, D.A., Lee,
L.E. and Mackay, J. (2020). Weekly Doxorubicin and
Continuous Infusional Przemyslaw Kozminski, Pawel Krzysztof Halik, Raphael
Chesori and Ewa Gniazdowska. Overview of Dual-Acting Drug Methotrexate in
Different Neurological Disease, Autoimmune Pathologies and Cancer.
International Journal of Molecular Sciences, 21(10). https://doi.org/10.3390/ijms21103483.
Ho, M. Y., and Mackey, J. R. (2014). Presentation and Management of Docetaxel-Related Adverse Effect in Patients With Breast Cancer. Cancer Management And Research 6, 253-259. https://doi.org/10.2147/CMAR.S40601.
Huang,L., Zeng, L. and Chu, Z. (2018). Chemoresistance-Related Long Non Coding RNA Expression Profiles in Human Breast Cancer Cells. Molecular Medicine Reports, 18(1), 243-253. https://doi.org/10.3892/mmr.2018.8942.
Jasra, S. and Anampa, J. (2018).
Anthracycline Use For Early Stage Breast Cancer in the Modern Era (2018) A
Review. Current Treatment Options In Oncology. https://doi.org/10.1007/s11864-018-0547-8.
Khanna,
C., Rosenberg, M. and Vail, D.M. (2015).
A Review Of Paclitaxel and Novel Formulations Including Those Suitable for Use in
Dogs. Journal of Veterinary Internal Medicine 1006-1012. https://doi.org/10.1111/jvim.12596.
Kumar, P. Raza, K. Kaushik, L. and Malik, R. (2016). Role of Colloidal Drug Delivery Carriers In Taxane-Mediated Chemotherapy: A Review. Curr. Pharm. Des. 22, 5127-5143. https://doi.org/10.2174/1381612822666160524144926.
Mansfield, C. M. (1993). "A Review of the Etiology of Breast Cancer". Journal of the National Medical Association, 217-221.
Mcgrogan, B.T., Gilmartin, B. and Carney,
D.N. (2008). Taxanes, Microtubules and Chemoresistant Breast Cancer.
Biochim. Biophys. Acta 1785, 96-132. https://doi.org/10.1016/j.bbcan.2007.10.004.
Patt, D., Gauthier, M., Giordano, S. (2006).
Paclitaxel in Breast Cancer. Women Health, 2(1), 11-21. https://doi.org/10.2217/17455057.2.1.11.
Poi, M. J., Berger, M., and Lustberg, M. (2013).
Docetaxel- Induce Skin Toxicities in Breast Cancer Patients Subsequent to
Paclitaxel Shortage: A Case Series and Literature Review. Support Care Cancer
21(10), 2679-2686.
https://doi.org/10.1007/s00520-013-1842-3.
Ribas, A., Albanell, J., Sole-Calvo, L. A. (1998). Cyclophosphamide, Methotrexate and Chronic Oral Tegafur Modulated by Folinic Acid in the Treatment of Patients with Advanced Breast Carcinoma. 22, 878-885. https://doi.org/10.1002/(SICI)1097-0142(19980301)82:5%3C878::AID-CNCR12%3E3.0.CO;2-Y.
Seely, J. M. and Alhassan, T. (2018). Screening of Breast Cancer in 2018- What Should We Be Doing Today. Current Oncology, S115-S124. https://doi.org/10.3747/co.25.3770.
Shewach,
D. S. and Kuchta, R. D. (2009).
Introduction of Cancer Chemotherapeutics. Chem Rev 109(7), 2859-2861. https://doi.org/10.1021/cr900208x.
Song, L., Zhang, Y. and He, J. (2011). The Side Effects of
Docetaxel With Cyclophosphamide as Postoperative Adjuvant Chemotherapy for
Elderly Breast Cancer Patients. Chinese- German Journal of Clinical Oncology,
391-393. https://doi.org/10.1007/s10330-011-0815-6.
Stark, L., Tofthage, C. and Mcmillan, S.C.
(2012). The Symptoms Experience in Patients with Cancer. Journal of Hospice
and Palliative Nursing 14(1), 61-70. https://doi.org/10.1097/NJH.0b013e318236de5c.
Thorn, C. F., Oshiro, C. and Marsh, S. (2011). "Doxorubicin Pathways: Pharmacodynamics and Adverse Effects." Pharmacogenet Genomics (7) 440-446. https://doi.org/10.1097/FPC.0b013e32833ffb56.
Wang, L. Y., Xie, H., and Zhou, H. (2017). Efficacy of
Carboplatin-Based Preoperative Chemotherapy for Triple-Negative Breast
Cakkkmmincer. Saudi Medical Journal 38(1), 18-23. https://doi.org/10.15537/smj.2017.1.14969.
Yap,H. Y., Blumenchein, G. R., Tashima, C. K., And Wang, A. Y. (1979). High Dose Methotrexate For Advanced Breast Cancer. Cancer Treat Rep. 63(5), 757-761.
Zhang,
D., Yangg, R., Wang, S. and Dong, Z. (2014). Paclitaxel New Uses for an
Old Drug. Drug Design, Development and Therapy, 279-284. https://doi.org/10.2147/DDDT.S56801.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2022. All Rights Reserved.