
CONCENTRATION OF Cr, Mn, Ni, Pb, and Zn IN A POPULATION LIVING NEAR AN INDUSTRIAL AREA IN THE BRAZILIAN EASTERN AMAZON
Ronaldo Magno
Rocha 1,2,3 ![]()
![]() ,
Simone de Fátima Pinheiro Pereira 2,3
,
Simone de Fátima Pinheiro Pereira 2,3![]()
![]() , Daniel Pinheiro Nogueira 3
, Daniel Pinheiro Nogueira 3![]()
![]() ,
Pedro Moreira de Sousa Junior 3,4
,
Pedro Moreira de Sousa Junior 3,4![]()
![]() ,
Alan Marcel Fernandes de Souza 3,5
,
Alan Marcel Fernandes de Souza 3,5![]()
![]() ,
Hemilton Cardoso da Costa 2,3
,
Hemilton Cardoso da Costa 2,3![]()
![]() ,
Cléber Silva e Silva
3,5
,
Cléber Silva e Silva
3,5![]()
![]() ,
Davis Castro dos Santos 3,6
,
Davis Castro dos Santos 3,6![]()
![]() ,
Thiago de Melo e Silva 2,3
,
Thiago de Melo e Silva 2,3![]()
![]()
1 Central Laboratory of the Pará Health
Department. Augusto Montenegro Avenue, 524, Parque Guajará, Belém, PA, Brazil
2 Chemistry Graduate Program, Federal
University of Pará. Augusto Correa Street, S/N, Guamá,
Belém, PA, Brazil
3 Environmental and Analytical Chemistry
Laboratory, Federal University of Pará. Augusto Correa Street, S/N, Guamá, Belém, PA, Brazil
4 Federal Rural University of the Amazon. Barão de Capanema Avenue S/N, Caixa D'Água, Capanema, PA, Brazil
5 Federal Institute of Education, Science, and Technology of Pará. Almirante Barroso Avenue, 1155, Marco, Belém, PA, Brazil
6 Federal University of
Pará - Altamira Campus. Coronel José Porfírio Street,
2515, São Sebastiao, Altamira, PA
|
|
ABSTRACT |
||
|
In Barcarena, several industries are in operation, some of these industries generate highly toxic by-products, which end up influencing the social, economic, and health conditions of the residents. This study aimed to evaluate the exposure of an amazonian population to the elements Cr, Mn, Ni, Pb, and Zn using hair as a bioindicator. The results showed the average hair contents of Cr (2.5±1.5 µg g-1), Mn (15.5±12.3 µg g-1), Ni (5.4±9.0 µg g-1), Pb (18.7±15.4 µg g-1), and Zn (274±227 µg g-1) in the studied residents were higher than the averages of the elements in other countries population. The highest concentrations of Ni, Pb, and Zn were detected in children under 11 years old. Cr stood out for presenting the highest levels in the 21 to 30 years old group and Mn presented a higher concentration range for the 11 to 20 years old group. Cr showed a significant correlation with age (0.901; p=0.014) in the group of children (age <11 years). |
|||
|
Received 17 October 2022 Accepted 18 November 2022 Published 02 December 2022 Corresponding Author Alan
Marcel Fernandes de Souza, alan.marcel@ifpa.edu.br
DOI10.29121/granthaalayah.v10.i11.2022.4867 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2022 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Environmental Exposure, Population
Health, Toxic Elements |
|||
1. INTRODUCTION
Toxic elements enter the ecosystem by natural and anthropogenic means, such sources include natural weathering, mining, soil erosion, domestic sewage, pesticide application, effluents, and atmospheric industrial emissions, among others. Several input sources of toxic elements from industrial processes can be responsible for population exposure, such as fossil fuel burning, lack of structure and waterproofing of tailings tailings basin, and non-treatment of effluents with high levels of toxic elements, etc. Zhuang et al. (2014).
The contamination of exposed populations to toxic elements occurs mainly through ingestion of contaminated water, food consumption, and air inhalation. To analyze toxic elements in the environmental biomonitoring of populations several types of matrices can be employed, such as urine and blood that show recent exposure. However, the determination of the elements in the blood does not necessarily reflect the current load of the organism as they undergo homeostatic mechanisms that instantly balance the concentration of elements and the concentration ranges are narrow Özkara and Akyl (2018).
Hair or nail analysis is potentially useful for an estimate of chronic population exposure phenomena caused by toxic elements in an industrial area. Several studies have reported the use of hair analysis to obtain information on environmental exposure to toxic elements. Hair is used as a biomarker of long-term chronic exposure with advantages over body fluids such as hair sampling is non-invasive, does not present storage problems, does not require special care as cooling, hair is a highly mineralized tissue, and the concentration of the minerals is about 10 times higher than in blood, plasma, or urine Skalny et al. (2017).
Minerals found in hair originate from the blood and are linked by hair follicle proteins. The irreversible incorporation of elements in the hair is a part of the excretory mechanism for toxic element elimination. The exposure of individuals to toxic elements using hair as a bioindicator can be influenced by several factors such as age, place of residence, gender, and smoking habit Skalnaya et al. (2016).
The exposure of populations in the Brazilian Eastern Amazon to toxic elements due to mining and industrial activity was reported by Carvalho et al. (2009) who evaluated As, Cd, Pb, and Hg in the hair of residents in the municipality of Altamira, and Pereira et al. (2010) who evaluated As in the hair of residents of the city of Santana. Recently, in studies carried out by Queiroz et al. (2019) and Naka et al. (2020), high levels of Pb and Cd were found in the blood of individuals living in a community located near the industrial area of Barcarena in the state of Pará.
The industries that process ores in the city of Barcarena, mainly the kaolin and bauxite processing industries, generate dangerous tailings that may contain high amounts of chemical elements, some carcinogens such as Cr, Pb, and Ni. Through several environmental disasters caused by the overflow of tailings basins these industries daily discharge effluents without treatment for metals in the regional rivers. The frequent input of these products in the environment can cause contamination of rivers, contamination of the groundwater table with consequent contamination of drinking water, loss of local biodiversity, loss of life quality, with risks to the population health Oliveira et al. (2020).
Considering that human hair provides retrospective information on the exposure of individuals, the purpose of this work was to study the level of exposure of the sample population to the elements Cr, Mn, Ni, Pb, and Zn, and the age effect on the concentration in human hair having as an anthropogenic factor the presence of the industrial pole installed in Barcarena.
2. MATERIAL AND METHODS
2.1. STUDY AREA
Barcarena Figure 1 is located at Latitude 1° 31' 8' 'South and Longitude 48° 37' 1'' West, on the banks of the Pará River, an important river in the region, near Belém, capital of the state of Pará in the Brazilian Eastern Amazon. Barcarena has an estimated population of 127,027 inhabitants in an area of 1,310,340 km². Barcarena, from the 1980s, began a history linked to the industrialization of aluminum and other industrial activities IBGE (2022).
At the margin of this industrial development process, there are several traditional communities, especially Curuperé, Dom Manuel, Vila Nova, Vila do Conde, and Itupanema, which for years have been affected by socio-environmental problems due to the lack of territorial ordering that causes the pairing of the villages with the tailing’s basins, increasing the risk to public health.
Figure 1
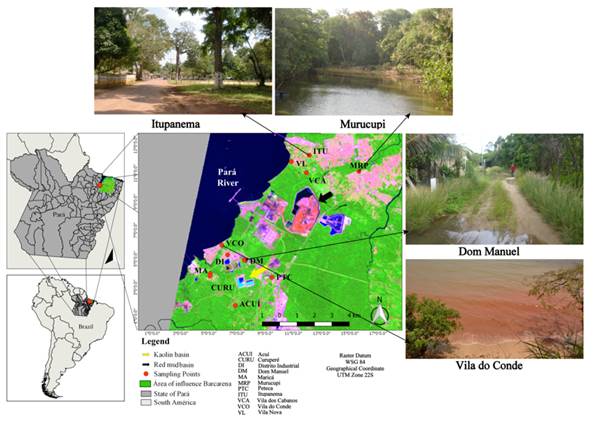
|
Figure 1 Map Illustrative Showing the Study Area Source Maps QGIS, INPE, Photos: LAQUANAM. |
2.2. SAMPLING AND COLLECTION
In this study,
all the ethical procedures foreseen in the Declaration of Helsinki and
Brazilian legislation were carried out, including the Informed Consent Form
(ICF) according to the protocol of the National Research Ethics Commission
(CONEP) of the National Health Council linked to the Brazilian Ministry of
Health and Internal Ethics Committee of the Federal University of Pará. For hair collection, authorization was initially obtained from the CONEP
(CAAE: 40012814.6.0000.0018) to research human beings. The research
participants were informed about the research objectives, after signing the
Informed Consent Form (ICF), they were referred to complete the questionnaire
and to collect their hair. All residences visited were georeferenced and the
hair sample extraction was limited to only one volunteer member per household
to which the ICF was issued.
Ninety
people (34 male and 56 female gender) who live near the industrial center of Barcarena-PA were
selected. The main filter for the selectable individual was the age group. The age was classified as following: Group 1- under 11 years old; Group 2- from 11 to 20 years old; Group 3 - from 21 to 30 years old, Group 4 - from 31 to 40 years old, Group 5 - from 41 to 50 years old, Group 6 - from 51 to 60 years old, and Group
7 - over 60 years old, including the residents of the Acuí,
Curuperé, Industrial District, Dom Manoel, Itupanema, Maricá, Murucupi, Peteca, Vila do Conde, Vila Nova, and Vila dos Cabanos communities, in the rural and urban area of Barcarena. The logic established in the research for the
chosen age groups followed the one established by Sukumar (2011) and Yuen et al. (2018).
For
the sampling design, a stratified random sampling methodology was used in order
to obtain a statistically representative set. This strategy was used because of
the cross-sectional study implemented in which collections were interspersed in
different locations of the Brazilian Amazon but with similar exposure patterns.
Noreen et al. (2020) conducted their research in the same way,
when working with cross-sectional studies.
The collection of capillary material was performed
according to the guidelines proposed by the National Human Exposure Assessment
Survey NHEXAS (2011) and
endorsed by authors such as Wang et al. (2019) and Li et al. (2020). Thus, the samples were extracted from the nape, as it is the area with
the largest amount of hair even in bald people. It is a consensus among the
researchers that the amount collected should be approximately 1 to 2 g of hair,
with the aid of stainless steel or titanium scissors, in a maximum interval of
1 cm in length so that there is no aesthetic damage.
2.3. SOCIOCULTURAL QUESTIONNAIRE
A research questionnaire (additional information) was adapted and adopted, based on literature review, to collect information on the perception of residents regarding their social conditions, health status, personal hygiene, food sanitation, and water uses and treatment, as suggested by Li et al. (2020) and Giné-Garriga et al. (2013).
2.4. DIGESTION OF SAMPLES
The collected samples were treated as recommended by the IAEA (1976). Authors, such as Wang et al. (2019) and Ali et al. (2017), report that the analytical protocol with the most effective result is based on successive 10-minute washes in acetone, followed by washes with deionized water and ending with acetone. Still based on their methodological procedures, each sample was filtered, dried in a laminar flow hood, and sent for digestion.
For sample opening procedure, the acid digestion method
was
employed with the aid of a microwave from the Provecto
Analítica brand, model DGT 100 Plus. This procedure
was adopted based on similar analyzes conducted by
researchers, such as Carvalho et al. (2009) and Pereira et al. (2010). It was
used 0.3 g of previously treated hair. In Teflon tubes, the following mixing
ratio was established: hair sample, 1.5 ml of supra pure 65 % nitric acid, and
0.25 ml of 30 % hydrogen peroxide PA. The program recommended by the microwave
manufacturer establishes a heating ramp. After cooling, the solutions were
filtered and transferred to polyethylene containers for further analytes
quantification.
2.5. Analytical Instrumentation
The metals Cr, Mn, Ni, Pb, and Zn were quantified by the optical emission spectrometry with inductively coupled plasma (ICP-OES), with the potential for simultaneous multi-element analyses, brand Varian and model Vista Pro, and based on U.S. Environmental Protection Agency Method nº 6010D (SW-846) (EPA, 2014).
The method linearity study was performed using an
analytical curve for each element in a specific wavelength (nm). Standard
solutions were prepared by diluting a multi-element solution with a 1M HNO3
solution that was used as a calibration blank several times (15 measures) to
calculate the detection limits (0.01 (Cr), 0.01 (Mn), 0.06 (Ni), 0.06 (Pb) and 0.02
(Zn) µg g-1) and quantification limits (0.04 (Cr), 0.04 (Mn), 0.18
(Ni), 0.18 (Pb) and 0.13 (Zn) µg g-1).
The investigation of accuracy and precision was
determined by carrying out ten determinations for various samples of the
certified reference for human hair (NCS DC 73347) from the China National
Analysis Center for Iron and Steel with recoveries of
101.2 % (Cr), 105.3 % (Mn), 90.3 % (Ni), 98.2 % (Pb) and 92.2 % (Zn).
2.6. STATISTICS
The
analytical results were treated statistically with the aid of the software Statistica 8.0® (Stat Soft. INC). The
correlation analysis was performed using Pearson's correlation coefficient (r).
In the box plot study, the median was used in the range of 25 to 75%. All
statistical tests performed were significant for a probability value of less than
5% (p <0.05).
3. RESULTS AND DISCUSSION
3.1. COMPARISON OF RESULTS
The descriptive statistics of the geral results of the hair elements of individuals residing
in Barcarena are shown in Table 1.
Table 1
|
Table 1 General Descriptive Statistics of Hair Elements (N=90) (µg g-1) |
|||||
|
Statistics |
Cr |
Mn |
Ni |
Pb |
Zn |
|
Mean |
2.50 |
15.5 |
5.40 |
18.7 |
274 |
|
Standard deviation |
1.47 |
12.3 |
8.97 |
15.4 |
227 |
|
Coefficient of variation (%) |
58.8 |
79.4 |
168 |
82.2 |
82.8 |
|
Minimum |
<0.039 |
<0.035 |
<0.181 |
3.77 |
<0.128 |
|
Maximum |
10.5 |
82.6 |
84.0 |
81.2 |
1137 |
|
N: Number of samples |
|||||
The average of all evaluated elements was in the
following decreasing order of concentration: Zn> Pb> Mn> Ni> Cr. Regarding the limits, it was possible to
infer the existence of a high range of values, represented by the coefficient
of variation (CV) of the concentration ranges 58.8 % (Cr) - 168 % (Ni), indicating that the matrix presents
great sample variability, typical behavior of the
non-normal distribution.
The average metals values
are above the standards established compared to the mean concentration of elements in
the hair of people in other countries Table 2.
Cr was 806 % above the
average found for other places. Tanneries workers with high
concentrations of Cr were studied by Kamran et al. (2014). The authors showed that individuals suffer from different diseases,
such as increased blood pressure, skin infection, jaundice, respiratory
disorder, etc. due to exposure to Cr. Due to the
presence of oxygen in excess in the environment, Cr+3 is oxidized to
Cr+6, which is extremely toxic and highly soluble in water and can
easily pass through the cell membrane. Cr+6, due to its mutagenic
properties, is considered a group 1 carcinogen by the International Agency for
Research on Cancer ATSDR (2012).
Mn was 3039 % above the average found for other places. The
high toxicity of Mn is well documented in numerous studies carried out in
workers in the mining, welding, and ferroalloy industries, and in other
occupational environments with a high level of exposure to Mn. Environmental
exposures to Mn occurring at lower levels and more continuous than occupational
exposures have become a public health concern, particularly regarding
vulnerable populations Röllin and Nogueira, (2011).
Park (2013) reviewed the neurotoxic effects of Mn and concluded that there is a
relationship between neurobehavioral deficit and Parkinsonism in workers
subjected to exposure to Mn in the air. Occupational and environmental exposure
to airborne Mn has been associated with neurobehavioral deficits in adults and
children Riojas-Rodríguez et al. (2010).
Table 2
|
Table 2 Elements’ Ccomparison of the Mean Concentration in the Hair of People in Other Countries (mg g-1) |
||||||
|
Cr |
Mn |
Ni |
Pb |
Zn |
References |
|
|
Brazil (Barcarena) |
2.50 |
15.5 |
5.40 |
18.7 |
274 |
Current study |
|
Brazil (Rio de Janeiro) |
NA |
0.62 |
0.41 |
6.4 |
189 |
Carneiro et al. (2002) |
|
Bolivia |
0.07 |
0.56 |
0.13 |
2.3 |
85.3 |
Barbieri et
al. (2011) |
|
Canada |
0.20 |
0.07 |
0.23 |
0.41 |
162 |
Goullé et al. (2005) |
|
China
(Tonglu) |
0.41 |
0.83 |
0.20 |
1.11 |
168 |
Luo et al. (2014) |
|
Italy (Palermo) |
0.11 |
0.31 |
0.55 |
1.01 |
189 |
Dongarrà et al. (2011) |
|
Italy (Rome) |
0.07 |
0.35 |
1.49 |
7.1 |
150 |
Senofonte et al. (2000) |
|
Italy
(Sardinia) |
0.16 |
0.32 |
0.60 |
0.98 |
203 |
Varrica et al. (2014) |
|
Philippines |
0.31 |
1.62 |
2.1 |
4.8 |
238 |
Sera et al. (2002) |
|
Poland |
1.2 |
0.73 |
0.95 |
3.1 |
230 |
Chojnacka et al. (2005) |
|
Spain
(Madrid) |
0.34 |
0.23 |
0.27 |
0.86 |
153 |
Gonzalez-Munoz
et al. (2008) |
|
Spain
(Madrid) |
0.35 |
0.32 |
0.55 |
0.77 |
110 |
Ballesteros et al. (2017) |
|
Taiwan |
0.14 |
0.23 |
0.29 |
0.39 |
175 |
Skalny et al. (2018) |
|
NA = Not
available |
||||||
Ni was 831 % above the average found for other places. Generally, Ni
occurs at very low levels in the environment, being the food the main source of
exposure to this metal. Air inhalation, water consumption, and contaminated
food ingestion can be routes of exposure to Ni ATSDR (2005a). The exposure of a
child still in the fetus to Ni can occur through the
transfer of Ni present in the mother's blood to the blood of the fetus and during breastfeeding.
Ni is a carcinogenic element in the respiratory tract, and it has been shown
that occupational exposure to Ni predisposes man to lung, larynx, and nasal
cancer ATSDR (2005a).
Pb was 766 % above the average found for other places. Among the
toxic metals, Pb is the most present in the environment. This element has no
known nutritional, biochemical, or physiological function. As there is no
demonstrated biological need, Pb is recognized by the World Health Organization
(WHO) as one of the most dangerous chemical elements to human health IARC (2006).
The effect of relatively low
exposure on cognitive and behavioral development in
children is extremely troubling, bioaccumulating with the metal's half-life.
Recently, special attention has been given to epidemiological studies aimed at
the possible neurotoxic effects of Pb in children, especially in those with behavioral disorders Blaurock-Busch et al. (2011).
Zn was 160 % above the average found for other places. Zinc is an essential trace element for humans, as it is strongly involved
in nutritional status, antioxidant systems, immune system, cell division, and
protein synthesis. Even though it is considered an important nutrient, both its
excess and its deficiency are the cause of several problems related to human
health ATSDR (2005b).
Zn in excess is considered an
environmental pollutant and can cause health problems, such as coronary artery
disease and sideroblastic anemia. Also, can cause
gastrointestinal irritation, stomach cramps, nausea, vomiting, damage to the
adrenal gland, damage to the pancreas, and decrease levels of high-density
lipoproteins (HDL) ATSDR et al. (2005b).
The presence of high levels of
elements Cr, Ni and, Pb in the hair of Barcarena
residents may indicate an anthropic origin of the element since it is not
naturally present in the amazonian environment
The contribution of
effluents from the tailings basin of industries located in the Industrial Pole
of Barcarena to the rivers in the region may be one
of the causes of the population's exposure to Cr, Mn, Ni, Pb and Zn, and other
elements Wang and Yang (2016).
In addition to the
contribution of effluents from the tailing’s basin in Barcarena,
the emission of particulate matter into the atmosphere, which occurs widely at
the industrial pole, may expose the residents to chemical elements Marcias et al. (2018). These emissions are also combined with
the water vapor that ends up returning to the surface in the form of rain and
incorporating itself into the soil, rivers, inside the houses, as well as on
plants surfaces, which can potentially contribute to the presence of the
elements found in the hair of the evaluated people Krupnova et al. (2020).
3.2. AGE EFFECT
There is a great difficulty in establishing
reference values for the elements in hair by age groups because of the effect
of variables, such as eating habits and characteristics of the sampled
population such as age, place of residence, gender, etc.
Table 3 shows the concentrations of metals in human hair of the resident populations in Barcarena city according to age groups.
The age group 1 (<11 years
old), 3 (21-30 years old) and 4 (31-40 years old) presented the following
decreasing order of concerning the concentration of the evaluated elements:
Zn> Pb> Mn> Ni> Cr. The age group 2 (11-20 years old), 5 (41-50
years old), 6 (51-60 years old) and 7 (>60 years old) presented the
following decreasing order of concerning the concentration of the evaluated
elements: Zn>Mn>Pb>Ni>Cr.
In group 1 (<11 years old),
the elements Ni, Pb, Zn had the highest concentrations.
In group 3 (21-30 years old)
the Cr had the highest concentrations and in group 5 (41-50 years old) the Mn
had the highest concentrations.
The lowest
concentrations of Cr and Ni, were found in the
group 7 (>60
years old). Skalnaya et al. (2016) reported that older individuals have significantly reduced Cr content in
their hair compared to younger individuals. Cr concentration intervals in all
age groups were higher than the values observed by Sazakli and Leotsinidis
(2017).
For Mn, the lowest
concentration was in the group 4 (31-40 years old), for Pb, the lowest
concentration was in the group 6 (51-60 years old) and Zn in the group 2 (11-20
years old).
Peña-Fernández et al. (2016) evaluated the content of Cr and other chemical elements in children from
the city of Alcalá de Henares (Spain). The children
were divided into age groups. The authors found that Cr, in the group of 6-9
years old was significantly affected by the area of residence, having an
average concentration range in this group, which is below those found in this
study.
The lowest variability of the
Cr was in the 21-30 age group, with three anomalous results and the highest
variability was in the 31-40 age group Figure 2.
The presence of high levels of
Cr in the hair of Barcarena residents in all age
groups may indicate an anthropic origin of the element since it is not
naturally present in the amazonian environment.
Mn had its lowest variability in the age group from
31 to 40 years without anomalous results. Its greatest variability was in the
group from 41 to 50 years old with one anomalous result Figure 3.
Table 3
|
Table 3 Concentrations of Metals in Human Hair of the Resident Populations in Barcarena City According to Age Groups (µg g-11) |
||||||
|
Group (Age) |
Mean |
Median |
SD |
Minimum |
Maximum |
N |
|
1 (<11) |
|
|
|
|
|
|
|
Cr |
2.36 |
2.18 |
1.11 |
<LOQ |
5.33 |
18 |
|
Mn |
13.9 |
13.1 |
9.27 |
<LOQ |
43.4 |
18 |
|
Ni |
8.82 |
3.43 |
19.0 |
<LOQ |
84.0 |
18 |
|
Pb |
28.5 |
23.6 |
20.4 |
3.77 |
71.9 |
18 |
|
Zn |
354 |
298 |
283 |
<LOQ |
1103 |
18 |
|
2 (11-20) |
Mean |
Median |
SD |
Minimum |
Maximum |
N |
|
Cr |
2.31 |
2.20 |
1.02 |
1.10 |
4.70 |
11 |
|
Mn |
19.0 |
13.6 |
21.8 |
5.13 |
82.6 |
11 |
|
Ni |
4.79 |
4.71 |
3.66 |
1.27 |
14.8 |
11 |
|
Pb |
16.9 |
11.6 |
13.0 |
5.78 |
51.0 |
11 |
|
Zn |
237 |
180 |
182 |
107 |
753 |
11 |
|
3 (21-30) |
Mean |
Median |
SD |
Minimum |
Maximum |
N |
|
Cr |
3.25 |
2.36 |
2.91 |
<LOQ |
10.5 |
11 |
|
Mn |
12.8 |
11.7 |
6.90 |
<LOQ |
27.4 |
11 |
|
Ni |
4.42 |
4.14 |
2.56 |
<LOQ |
8.46 |
11 |
|
Pb |
16.1 |
10.7 |
13.9 |
3.86 |
44.5 |
11 |
|
Zn |
286 |
160 |
326 |
0.09 |
1138 |
11 |
|
4 (31-40) |
Mean |
Median |
SD |
Minimum |
Maximum |
N |
|
Cr |
2.33 |
2.18 |
1.30 |
<LOQ |
4.43 |
12 |
|
Mn |
10.4 |
9.34 |
4.51 |
0.06 |
16.3 |
12 |
|
Ni |
4.71 |
3.34 |
4.33 |
<LOQ |
14.2 |
12 |
|
Pb |
18.6 |
13.3 |
20.5 |
3.86 |
81.2 |
12 |
|
Zn |
260 |
248 |
164 |
<LOQ |
680 |
12 |
|
5 (41-50) |
Mean |
Median |
SD |
Minimum |
Maximum |
N |
|
Cr |
2.78 |
2.91 |
1.22 |
<LOQ |
4.98 |
13 |
|
Mn |
21.2 |
17.2 |
15.4 |
<LOQ |
55.9 |
13 |
|
Ni |
5.60 |
5.03 |
3.73 |
<LOQ |
13.6 |
13 |
|
Pb |
17.0 |
11.6 |
12.2 |
3.78 |
51.0 |
13 |
|
Zn |
248 |
206 |
179 |
<LOQ |
715 |
13 |
|
6 (51-60) |
Mean |
Median |
SD |
Minimum |
Maximum |
N |
|
Cr |
3.07 |
2.73 |
1.53 |
1.22 |
6.30 |
8 |
|
Mn |
13.0 |
14.0 |
5.82 |
4.36 |
20.1 |
8 |
|
Ni |
4.21 |
3.54 |
3.01 |
2.01 |
11.4 |
8 |
|
Pb |
12.8 |
11.2 |
4.70 |
7.91 |
18.8 |
8 |
|
Zn |
238 |
181 |
141 |
159 |
572 |
8 |
|
7 (>60) |
Mean |
Median |
SD |
Minimum |
Maximum |
N |
|
Cr |
1.94 |
2.07 |
0.71 |
<LOQ |
2.89 |
17 |
|
Mn |
17.1 |
14.7 |
12.2 |
<LOQ |
49.8 |
17 |
|
Ni |
3.44 |
3.07 |
2.17 |
<LOQ |
7.11 |
17 |
|
Pb |
15.2 |
13.8 |
9.28 |
3.84 |
36.9 |
17 |
|
Zn |
255 |
202 |
231 |
<LOQ |
1078 |
17 |
|
SD: Standard deviation, N: Number of
samples, <LOQ Below the Limit of quantification |
||||||
Riojas-Rodríguez et al. (2010)
studied the environmental exposure of Mn in children who lived in the vicinity
of the mining district of Molango, Mexico. The
authors found Mn in the children's hair. The same age group from Bacarena presented Mn levels higher than those from Molango, which confirms that the average levels of Mn in Barcarena are compared to values found in places of high
exposure to Mn.
Figure 2
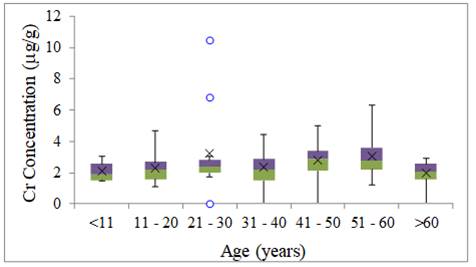
|
Figure 2 Cr Concentration of Metals in Human Hair for Age Group (µg g-1) |
According to Nitin and Bowman (2018), by
accumulating in the brain, Mn can trigger dysfunction of neurotransmitters,
glutamate, gamma-aminobutyric acid, and dopamine, which could suggest a
possible association of Mn from water intake to the increased risk of
neurobehavioral diseases for 6-18 years old children Liu et al. (2020). For
groups over 18 years old, hair is the most reliable indicator of Mn exposure Oulhote et al. (2014). In group 5 (41-50 years old), the Mn had the highest concentrations in Barcarena city, which reinforces the results found by the
authors and indicates greater care in relation to this age group (over 18
years old) in health research.
Figure 3
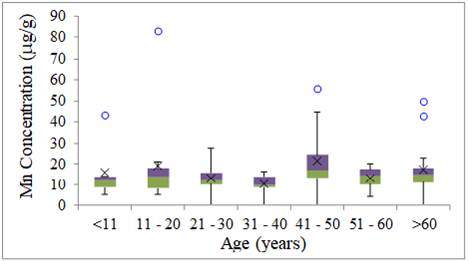
|
Figure 3 Mn Concentration of Metals in Human Hair for Age Group (mg g-1) |
The highest
concentration of Ni was verified in the children's group, and the lowest level
was found in the oldest group. These results agree with those found by Fiore et al. (2020), as the average nickel levels decreased in
comparison with the reference values related to age. Ni intervals, for all age
groups, were higher than the levels reported by Sazakli and Leotsinidis
(2017).
Ni variability was
small in all age groups with the exception of the
group of children (<11 years) with one anomalous result Figure 4.
Figure 4
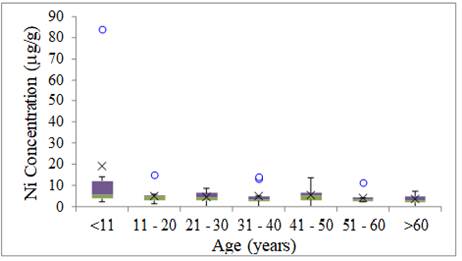
|
Figure 4 Ni Concentration of Metals in Human Hair for Age Group (mg g-1) |
Skalnaya et al. (2016) studied the change
in trace elements content in hair in relation to age groups using 10-year
intervals. In this study, the authors concluded that the maximum Ni content in
the hair was observed in the youngest age group, corroborating with the results
of this study.
When the results of the survey are compared with Senofonte et al. (2000) and Nouioui et al. (2018) who determined
Reference Values (RVs) in hair samples in various age groups, it was found that
the mean Ni contents determined are above the values proposed by the mentioned
authors.
The smallest variability of
results for Pb was in the group of 31 to 40 years with one anomalous result,
the greatest variability was in the group of children without anomalous results
Figure 5.
Li et al. (2020) evaluated Pb in the hair of 259 individuals residing in the city of Huludao (China) and showed that the highest concentrations
of Pb were detected in the 0-15 years old group, which was significantly higher
than those in other age groups (p<0.05), showing similarities to the results
observed in this study. Wang et al. (2019) explain that children generally absorb a greater amount of Pb than
adults due to a higher level of physical and metabolic activity, and greater Pb
absorption and retention in the gastrointestinal tract. The data also suggested
that children consumed greater amounts of food than adults in relation to body
weight, which results in children being potentially more likely to be exposed
to Pb in food than adults Skalny et al. (2018).
Peña-Fernández et al. (2016) reported that infants, children, and young people should be a target
audience in population surveys as they are more susceptible to environmental
pollutants than adults. They also point out that in these age groups the
metabolism factor and higher absorption rates in the gastrointestinal tract are
favorable conditions, thus they become more
susceptible to pollution in general.
The lowest variability of
results for Zn occurred in the group of 51 to 60 years with one anomalous
result. The greatest variability for Zn occurred in the groups of children
(<11 years) and in the group from 21 to 30 years with one anomalous result Figure 6.
Figure 5
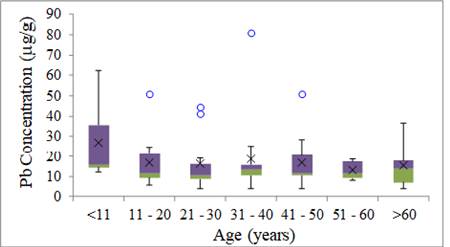
|
Figure 5 Pb Concentration of Metals in Human Hair for Age Group (mg g-1) |
The Zn levels in Barcarena were higher than was obtained by Skalny et al. (2018) in the hair of children with ASD (Autism Spectrum
Disorder) for two age groups, respectively, from 2 to 5 years old. Fiore
et al. (2020) reported that the mean levels of zinc decreased in comparison
with the reference values related to age.
The Zn intervals observed in
this work were above the reference values suggested by Sazakli and Leotsinidis
(2017) for individuals residing in some European countries.
Figure 6
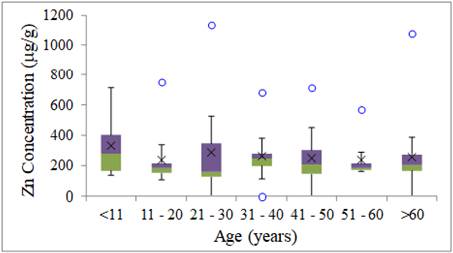
|
Figure 6 Zn Concentration of Metals in Human Hair for Age Group (mg g-1) |
Ali et al. (2019) investigated the hair concentration of eleven trace elements of
residents of Hefei, China in different age groups and found a high level of Zn
in the age group between 21 and 30 years old in men, and between 31 to 40 years
old in the case of women. The authors report that Zn concentration is commonly
low in the elderly group compared to middle-aged individuals, which may be due
to low protein absorption in the older age group, agreeing with the data
obtained in this study.
Li et al. (2020) noticed that Zn concentrations were higher than the average
concentration considered normal for human hair. Zn concentrations in the 25-35
years old group were statistically higher than in the 0-15, 35-45, and >55
years old groups (p <0.05), which differ from those found in this stud, as
the <11 years old group presented the highest levels of Zn.
3.2.1. Correlation Age effect and
Analysis of Variance
The Table 4 explains correlations between the metals Cr, Mn, Ni, and Zn in all age groups. Such a characteristic was already expected since authors such as Filippini et al. (2018) and Caparros-Gonzalez et al. (2019) state that mentioned metals are part of the group of essential nutrients for the maintenance of human organisms.
Cr showed a significant correlation with age (0.901; p=0.014) in the group of children (age
<11 years). Among the elements researched the Cr correlated with Mn in the
21-30, 31-40, and 51-60 age groups. There was also a correlation between Cr and
Pb in the group from 31 to 40 years old and in the elderly group (>60 years
old). Mn and Ni were correlated (p<0.05)
in the groups of children (<11 years) and in the group of adolescents (11 to
21 years). Mn also correlated with Zn in the 41-50 age group. Pb correlated
with Zn in the children's group (<11 years) and in the elderly group (>60
years). Ni correlated with Zn in the 51 to 60 years old group and in the
elderly group (>60 years old), it also correlated with Pb in this group. Pb
correlated with Zn in the group of children (<11 years) and in the elderly
group (>60 years).
Regarding the identified
correlations, it was possible to verify some important interactions between the
analyzed elements. For better interpretation, the
analysis will be performed from 2 groups: Essential Elements (EE) and Potential
Toxic Elements (EPT), as suggested by Lučić et al. (2022).
Lv et al. (2021) state that in biological systems, most essential metals combine with
proteins to form metalloproteins, which played an important role in the
enzymatic system for transporting nutrients to specific locations in organisms.
For example, during various biological processes, manganese acts as a coenzyme,
such as a macronutrient metabolism, free radical defense
systems, bone formation, and in the brain, neurotransmitter synthesis and
ammonia removal Erikson and Aschner (2019).
In relation to EPTs,
specifically Cr and Pb, the analysis reflects an inverse process of
bioconcentration in the organism. This behaviour can be explained by research
developed by Lv et al. (2021) in which it is reported that essential metals antagonize the effects of
toxic metals on the human body, attenuating their effects. The authors also
emphasize that there is a nutritional loss across age groups and that the
sixty-year-olds are the most affected mainly by the metabolic changes that
occur in the human body, such as obesity, diabetes, coronary heart disease, and
a diet with a nutritional deficit, which promotes elimination. The exponential
growth of these EE's through the body's natural
excretion processes Afridi et al. (2011).
When absorbed in concentrations
above the recommended, these metals have very similar physiological mechanisms
starting by the liver that undergoes an inflammatory process causing an
increase in the production of Aspartate AminoTransferase
(AST) and (Alanine AminoTransferase) ALT by the
hepatocytes causing its gradual degeneration, even genomic damage due to the
induction of antioxidant enzymes impairing the defense
mechanisms by the excess production of reactive oxygen species (ROS)
compromising the guanine of the DNA molecule González-Rendón et al. (2018), Monga et al. (2022).
As the variables present
parametric behavior, one-way ANOVA was used to
identify the analysis of variance of the Barcarena
age groups according to the content of trace metals.
Table 4
|
Table 4 Correlation of Elements Between Age Groups |
|||||||||||||
|
<11 |
Age |
Cr |
Mn |
Ni |
Pb |
Zn |
11-20 |
Age |
Cr |
Mn |
Ni |
Pb |
Zn |
|
Age |
1,000 |
|
|
|
|
|
Age |
1,000 |
|
|
|
|
|
|
Sic. |
- |
|
|
|
|
|
Sic. |
- |
|
|
|
|
|
|
Cr |
0,901 |
1,000 |
|
|
|
|
Cr |
0,016 |
1,000 |
|
|
|
|
|
Sic. |
0,014 |
- |
|
|
|
|
Sic. |
0,963 |
- |
|
|
|
|
|
Mn |
-0,449 |
-0,486 |
1,000 |
|
|
|
Mn |
-0,029 |
0,309 |
1,000 |
|
|
|
|
Sic. |
0,372 |
0,328 |
- |
|
|
|
Sic. |
0,932 |
0,356 |
- |
|
|
|
|
Ni |
-0,287 |
-0,364 |
0,980 |
1,000 |
|
|
Ni |
0,147 |
0,155 |
0,907 |
1,000 |
|
|
|
Sic. |
0,582 |
0,478 |
0,001 |
- |
|
|
Sic. |
0,667 |
0,649 |
0,000 |
- |
|
|
|
Pb |
-0,618 |
-0,627 |
0,467 |
0,335 |
1,000 |
|
Pb |
-0,392 |
0,507 |
0,356 |
0,231 |
1,000 |
|
|
Sic. |
0,191 |
0,183 |
0,350 |
0,517 |
- |
|
Sic. |
0,234 |
0,111 |
0,282 |
0,494 |
- |
|
|
Zn |
-0,505 |
-0,483 |
0,234 |
0,123 |
0,919 |
1,000 |
Zn |
0,542 |
-0,094 |
0,036 |
0,226 |
-0,215 |
1,000 |
|
Sic. |
0,307 |
0,332 |
0,656 |
0,817 |
0,009 |
- |
Sic. |
0,085 |
0,784 |
0,915 |
0,505 |
0,525 |
- |
|
21-30 |
Age |
Cr |
Mn |
Ni |
Pb |
Zn |
31-40 |
Age |
Cr |
Mn |
Ni |
Pb |
Zn |
|
Age |
1,000 |
|
|
|
|
|
Age |
1,000 |
|
|
|
|
|
|
Sic. |
- |
|
|
|
|
|
Sic. |
- |
|
|
|
|
|
|
Cr |
0,305 |
1,000 |
|
|
|
|
Cr |
0,242 |
1,000 |
|
|
|
|
|
Sic. |
0,361 |
- |
|
|
|
|
Sic. |
0,448 |
- |
|
|
|
|
|
Mn |
-0,031 |
0,751 |
1,000 |
|
|
|
Mn |
0,219 |
0,634 |
1,000 |
|
|
|
|
Sic. |
0,928 |
0,008 |
- |
|
|
|
Sic. |
0,494 |
0,027 |
- |
|
|
|
|
Ni |
-0,534 |
-0,117 |
0,121 |
1,000 |
|
|
Ni |
-0,272 |
-0,017 |
0,423 |
1,000 |
|
|
|
Sic. |
0,091 |
0,731 |
0,723 |
- |
|
|
Sic. |
0,392 |
0,959 |
0,170 |
- |
|
|
|
Pb |
0,073 |
-0,197 |
-0,007 |
0,414 |
1,000 |
|
Pb |
-0,072 |
0,588 |
0,466 |
0,105 |
1,000 |
|
|
Sic. |
0,830 |
0,561 |
0,984 |
0,206 |
- |
|
Sic. |
0,824 |
0,045 |
0,127 |
0,746 |
- |
|
|
Zn |
-0,354 |
0,129 |
0,308 |
0,291 |
-0,120 |
1,000 |
Zn |
0,502 |
0,395 |
0,197 |
0,025 |
0,033 |
1,000 |
|
Sic. |
0,285 |
0,707 |
0,357 |
0,386 |
0,725 |
- |
Sic. |
0,096 |
0,203 |
0,540 |
0,938 |
0,920 |
- |
|
41-50 |
Age |
Cr |
Mn |
Ni |
Pb |
Zn |
51-60 |
Age |
Cr |
Mn |
Ni |
Pb |
Zn |
|
Age |
1,000 |
|
|
|
|
|
Age |
1,000 |
|
|
|
|
|
|
Sic. |
- |
|
|
|
|
|
Sic. |
- |
|
|
|
|
|
|
Cr |
0,311 |
1,000 |
|
|
|
|
Cr |
0,646 |
1,000 |
|
|
|
|
|
Sic. |
0,301 |
- |
|
|
|
|
Sic. |
0,083 |
- |
|
|
|
|
|
Mn |
-0,152 |
0,163 |
1,000 |
|
|
|
Mn |
0,492 |
0,737 |
1,000 |
|
|
|
|
Sic. |
0,620 |
0,595 |
- |
|
|
|
Sic. |
0,216 |
0,037 |
- |
|
|
|
|
Ni |
-0,200 |
0,446 |
0,088 |
1,000 |
|
|
Ni |
-0,213 |
0,146 |
0,432 |
1,000 |
|
|
|
Sic. |
0,512 |
0,126 |
0,775 |
- |
|
|
Sic. |
0,612 |
0,730 |
0,285 |
- |
|
|
|
Pb |
0,243 |
0,191 |
-0,032 |
0,020 |
1,000 |
|
Pb |
0,044 |
0,376 |
0,667 |
0,590 |
1,000 |
|
|
Sic. |
0,424 |
0,533 |
0,918 |
0,949 |
- |
|
Sic. |
0,917 |
0,359 |
0,071 |
0,124 |
- |
|
|
Zn |
-0,192 |
0,340 |
0,579 |
0,408 |
0,110 |
1,000 |
Zn |
-0,023 |
0,418 |
0,417 |
0,908 |
0,512 |
1,000 |
|
Sic. |
0,530 |
0,255 |
0,038 |
0,167 |
0,720 |
- |
Sic. |
0,957 |
0,302 |
0,304 |
0,002 |
0,194 |
- |
|
>60 |
Age |
Cr |
Mn |
Ni |
Pb |
Zn |
|
|
|
|
|
|
|
|
Age |
1,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Sic. |
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cr |
0,109 |
1,000 |
|
|
|
|
|
|
|
|
|
|
|
|
Sic. |
0,678 |
- |
|
|
|
|
|
|
|
|
|
|
|
|
Mn |
-0,017 |
0,175 |
1,000 |
|
|
|
|
|
|
|
|
|
|
|
Sic. |
0,948 |
0,502 |
- |
|
|
|
|
|
|
|
|
|
|
|
Ni |
-0,179 |
0,469 |
0,203 |
1,000 |
|
|
|
|
|
|
|
|
|
|
Sic. |
0,493 |
0,057 |
0,434 |
- |
|
|
|
|
|
|
|
|
|
|
Pb |
-0,088 |
0,541 |
0,376 |
0,514 |
1,000 |
|
|
|
|
|
|
|
|
|
Sic. |
0,736 |
0,025 |
0,137 |
0,035 |
- |
|
|
|
|
|
|
|
|
|
Zn |
0,224 |
0,448 |
0,041 |
0,555 |
0,658 |
1,000 |
|
|
|
|
|
|
|
|
Sic. |
0,387 |
0,071 |
0,875 |
0,021 |
0,004 |
- |
|
|
|
|
|
|
|
|
Sic. = Significant correlations, Marked p
<0.050 |
|||||||||||||
Based on the test, the metals
Cr (Fcalculated (1,268) <Fcritical (2,210), p=0,281), Mn (Fcalculated (1,214) <Fcritical (2,210), p=0,308), Ni (Fcalculated (0,620) <Fcritical (2,210), p=0,714), Pb (Fcalculated (1,746) <Fcritical (2,210), p=0,121) and Zn (Fcalculated (0,499) <Fcritical (2,210), p=0,807) showed similar behaviour in the age groups, indicating that there is no
direct correlation of the set of elements analyzed.
4. CONCLUSIONS
The levels of Cr, Mn, Ni, Pb,
and Zn in Barcarena are higher than the other
countries for all age groups evaluated. The Barcarena
population is exposed to the elements with changes associated with age in the
concentration of Ni and Pb. Ni, Pb and Zn in hair was higher in the group
<11 years old. The data obtained suggest that changes related to age in the
concentration of elements can contribute to the development of diseases related
to the presence of toxic elements. The data obtained suggest that
age-associated changes in the element’s concentration can contribute to the
development of diseases related to the presence of toxic elements. This finding
suggests a broad study on the health of individuals in these age groups,
especially in the age group < 11 years old. The contour maps showed that the
concentration of the elements in the Barcarena
population is higher close to tailings basin and that this proximity may be
influencing the increased exposure of these elements in the population studied.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
The authors thank the support of the Chemistry Graduate Program, Environmental and Analytical Chemistry Laboratory, Scholarship Federal University of Pará Augusto Fernando Souza de Oliveira, Secretariat of Health, and Environment of the State of Pará, for the help in samples analysis, Federal Prosecutor Bruno Valente, Mrs. Cleide Monteiro, Mr. Petronilo Progênio Alves, and Mrs. Maria do Socorro Costa da Silva local community leaders, for the help in samples collection.
REFERENCES
ATSDR - Agency for Toxic Substances and Disease Registry. (2005a). Toxicological Profile for Nickel. U.S. Department of Health and Human Services. Atlanta, Georgia, 397.
ATSDR - Agency for Toxic Substances and Disease Registry. (2005b). Toxicological Profile for Zinc. U.S. Department of Health and Human Services. Atlanta, Georgia, 352.
ATSDR - Agency for Toxic Substances and Disease Registry. (2012). Toxicological Profile for Chromium. U.S. Department of Health and Human Services. Atlanta, Georgia, 592.
Afridi, H. I., Kazi, T. G., Kazi, N. S., Kandhro, G. A., Baig, J. A., Shah, A. Q., Jamali, M. K., Arain, M. B., Wadhwa, S. K., Khan, S., Kolachi, N. F., and Shah, F. (2011). Chromium and Manganese Levels in Biological Samples of Pakistani Myocardial Infarction Patients at Different Stages as Related to Controls. Biological Trace Element Research, 142(3), 259-273. https://doi.org/10.1007/s12011-010-8773-3.
Ali, M. U., Liu, G., Yousaf, B., Abbas, Q., Munir, M. A. M.,and Fu, B. (2017). Pollution Characteristics and Human Health Risks of Potentially (Eco) Toxic Element (Ptes) in Road Dust from Metropolitan Area of Hefei, China. Chemosphere, 181,111-121. https://doi.org/10.1016/j.chemosphere.2017.04.061.
Ali, M. U., Liu, G., Yousaf, B., Ullah, H., Abbas, Q., Munir, M. A. M., and Irshad, S. (2019). Biomonitoring and Health Risks Assessment of Trace Elements in Various Age- and Gender-Groups Exposed to Road Dust in Habitable Urban-Industrial Areas of Hefei, China. Environmental Pollution, 244, 809-817. https://doi.org/10.1016/j.envpol.2018.10.084.
Ballesteros, M. T. L., Serrano, I. N., and Álvarez, S. I. (2017). Reference Levels of Trace Elements in Hair Samples from Children and Adolescents in Madrid, Spain. Journal of Trace Element Medicine Biology, 43, 113-120. https://doi.org/10.1016/j.jtemb.2016.12.010.
Barbieri, F. L., Cournil, A., Sarkis, J. E. S., Bénéfice, E., and Gardon, J. (2011). Hair Trace Elements Concentration to Describe Polymetallic Mining Waste Exposure in Bolivian Altiplano. Biological Trace Element Research, 139, 10-23. https://doi.org/10.1007/s12011-010-8641-1.
Blaurock-Busch, E., Amin, O. R., and Rabah, T. (2011). Heavy Metals and Trace Elements in Hair and Urine of a Sample of Arab Children With Autistic Spectrum Disorder. Maedica, 6, 247-257.
Caparros-Gonzalez, R. A., Giménez-Asensio, M. J.,
González-Alzaga, B., Aguilar-Garduño, C., Lorca-Marín, J. A., Alguacil, J.,
Gómez-Becerra, I., Gómez-Ariza, J. L., García-Barrera, T., Hernandez, A. F.,
López-Flores, I., Rohlman, D. S., Romero-Molina, D., Ruiz-Pérez, I., Lacasaña,
M. (2019). Childhood Chromium Exposure and Neuropsychological
Development in Children Living in Two Polluted Areas in Southern Spain.
Environmental Pollution, 252, 1550-1560. https://doi.org/10.1016/j.envpol.2019.06.084.
Carneiro, M. T. W. D., Silveira, C. L. P., Miekeley, N., and Fortes, L. M. C. (2002). Intervalos de Referência para Elementos Menores e Traço em Cabelo Humano para a População da Cidade do Rio de Janeiro - Brasil. Quim. Nova, 25, 37-45. https://doi.org/10.1590/S0100-40422002000100008.
Carvalho, A. S. C., Santos, A. S., Pereira, S. F. P., and Alves,
C. N. (2009). Levels of As, Cd, Pb and Hg Found in the Hair from People
Living in Altamira, Pará, Brazil : Environmental Implications in the Belo Monte
Area. Journal of the Brazilian Chemical Society, 20, 1153-1163. https://doi.org/10.1590/S0103-50532009000600022.
Chojnacka, K., Górecka, H., Chojnacki, A., and Górecki, H. (2005). Inter-Element Interactions in Human Hair. Environmental Toxicology Pharmacology, 20, 368-374. https://doi.org/10.1016/j.etap.2005.03.004.
Dongarrà, G., Lombardo, M., Tamburo, E., Varrica, D., Cibella,
F., and Cuttitta, G. (2011). Concentration and Reference Interval of
Trace Elements in Human Hair from Students Living in Palermo, Sicily (Italy). Environmental
Toxicology Pharmacology, 32, 27-34. https://doi.org/10.1016/j.etap.2011.03.003.
EPA - U.S. Environmental Protection Agency (2014). Method nº 6010D (SW-846) - Inductively Coupled Plasma-Atomic Emission Spectrometry Method.
Erikson, K. M., and Aschner, M. (2019). Manganese: Its Role in Disease and Health. Met. Ions Life Science. https://doi.org/10.1515/9783110527872-016.
Filippini, T., Cilloni, S., Malavolti, M., Violi, F., Malagoli, C., Tesauro, M., Bottecchi, I., Ferrari, A., Vescovi, L., and Vinceti, M. (2018). Dietary Intake of Cadmium, Chromium, Copper, Manganese, Selenium and Zinc in a Northern Italy Community. Journal of Trace Elements in Medicine and Biology, 50, 508-517. https://doi.org/10.1016/j.jtemb.2018.03.001.
Fiore, M., Barone, R., Copat, C., Grasso, A., Cristaldi, A., Rizzo,
R., and Ferrante, M. (2020). Metal and Essential Element Levels in Hair
and Association with Autism Severity. Journal of Trace Elements in Medicine and
Biology, 57, 99-103.
https://doi.org/10.1016/j.jtemb.2019.126409.
Giné-Garriga, R., Palencia, A. J. F., and Pérez-Foguet, A. (2013). Water-Sanititation-Hygiene Mapping : An Improved Approach for Data Collection at Local Level. Sci. Total Environment, 463-464, 700-711. https://doi.org/10.1016/j.scitotenv.2013.06.005.
Gonzalez-Munoz, M. J., Pena, A., and Meseguer, I. (2008). Monitoring Heavy Metal Contents in Food and Hair in a Sample of Young Spanish Subjects. Food Chemical Toxicology, 46, 3048-3052. https://doi.org/10.1016/j.fct.2008.06.004.
González-Rendón, E. S., Cano, G. G., Alcaraz-Zubeldia, M., Garibay-Huarte, T., and Fortoul, T. I. (2018). Lead Inhalation and Hepatic Damage : Morphological and Functional Evaluation in Mice. Toxicology and Industrial Health, 34(2), 128-138. https://doi.org/10.1177/0748233717750981.
Goullé, J. P., Loïc, M., Castermant, J., Neveu, N., Bonneau, L., Lainé, G., Bouige, D., and Lacroix, C. (2005). Metal and Metalloid Multi-Elementary ICP-MS Validation in Whole Blood, Plasma, Urine and Hair: Reference Values. Forensic Science International. 153, 39-44. https://doi.org/10.1016/j.forsciint.2005.04.020.
IAEA - International Atomic Energy Agency. (1976).
Activation Analysis of Hair
as an Indicator of Contamination of Man By Environmental
Trace Element Pollutants. Seibersdorf (Austria) : Agency's Laboratories, Analytical Quality Control
Services.
IARC (2006). Monographs on the Evaluation of Carcinogenic Risks to Humans. Inorganic and Organic Lead Compounds. 87.
IBGE Instituto Brasileiro de Geografia e Estatística. (2022). Informações da cidade de Barcarena. 2020.
Kamran, R., Abbas, M., and Sarwar,
S. (2014). Detection of Chromium
in Hair Samples of Male
Tannery Workers Near Gajju Matah. Asian Journal Agriculture Biology,
2, 148-151.
Krupnova, T. G., Rakova, O. V., Gavrilkina,
S. V., Antoshkina, E. G., Baranov, E. O., and Yakimova, O. N. (2020).
Road Dust Trace Elements Contamination, Sources, Dispersed Composition, and
Human Health Risk in Chelyabinsk, Russia. Chemosphere, 261, 127799. https://doi.org/10.1016/j.chemosphere.2020.127799.
Li, Y., Yu, Y., Zheng, N., Hou, S., Song, X., and Dong, W. (2020). Metallic Elements in Human Hair from Residents in Smelting Districts in Northeast China : Environmental Factors and Differences in Ingestion Media. Environmental Research, 182(108914), 1-10. https://doi.org/10.1016/j.envres.2019.108914.
Liu, W., Xin, Y., and Li, Q. (2020). Biomarkers of
Environmental Manganese Exposure and Associations With Childhood
Neurodevelopment: A Systematic Review and Meta-Analysis. Environmental Health,
19, 1-22. https://doi.org/10.1186/s12940-020-00659-x.
Luo, R., Zhuo, X., and Ma, D. (2014). Determination of 33
Elements in Scalp Hair Samples from Inhabitants of a Mountain Village of Tonglu
City, China. Ecotoxicology Environmental Safety, 104, 215-219. https://doi.org/10.1016/j.ecoenv.2014.03.006.
Lučić, M., Miletić, A., Savić, A.,
Lević, S., Ignjatovićd, I. S., and Onjia, A. (2022). Dietary Intake
and Health Risk Assessment of Essential and Toxic Elements in Pepper (Capsicum Annuum).
Journal of Food Composition And Analysis, 111(104598). https://doi.org/10.1016/j.jfca.2022.104598.
Lv, Y., Wei, Y., Zhou, J., Xue, K, Guo, Y., Liu, Y., Ju, A., Wu, B.,
Zhao, F., Chen, C., Xiong, J., Li, C., Gu, H., Cao, Z., Ji, J. S., and Shi, X.
(2021). Human Biomonitoring of Toxic and Essential Metals in Younger
Elderly, Octogenarians, Nonagenarians And Centenarians: Analysis of the Healthy
Ageing and Biomarkers Cohort Study (HABCS) in China. Environment International,
156, 106717. https://doi.org/10.1016/j.envint.2021.106717.
Marcias,
G., Fostinelli, J., Catalani, S., Uras, M., Sanna, A. M., Avataneo, G., Palma,
G. D., Fabbri, D., Paganelli, M., Lecca, L. I., Buonanno, G., and Campagna, M.
(2018). Composition of Metallic Elements and Size Distribution of Fine and
Ultrafine Particles in a Steelmaking Factory. International Journal
Environmental Research Public Health, 15(1192), 1-13. https://doi.org/10.3390/ijerph15061192.
Monga, A., Fulke, A. B., and Dasgupta, D. (2022). Recent Developments
in Essentiality of Trivalent Chromium and Toxicity of Hexavalent Chromium: Implications
on Human Health and Remediation Strategies. Journal of Hazardous Materials
Advances, 7 (100113), 1-18.
https://doi.org/10.1016/j.hazadv.2022.100113.
NHEXAS - National Human Exposure Assessment Survey. (2011). Quality Systems and Implementation Plan for Human Exposure Assessment. Region 5 Study. Revision 1, 2-8.
Naka, K. S., Mendes, L. C. S., Queiroz, T. K. L., Costa, B. N. S., Jesus, I. M., Câmara, V. M., and Lima, M. O. (2020). A Comparative Study of Cadmium Levels in Blood from Exposed Populations in an Industrial Area of the Amazon, Brazil. Science of the Total Environment, 698, 1-9. https://doi.org/10.1016/j.scitotenv.2019.134309.
Nitin, R., and Bowman, A. B. (2018). Connections Between
Manganese Neurotoxicity and Neurological Disease. Advances in Neurotoxicol., 2,
87-113. https://doi.org/10.1016/bs.ant.2018.03.001.
Noreen, F., Sajjad, A., Mahmood, K., Anwar, M., Zahra, M., and
Waseem, A. (2020). Human Biomonitoring of Trace Elements in Scalp Hair
from Healthy Population of Pakistan. Biological Trace Element Research 196,
37-46. https://doi.org/10.1007/s12011-019-01906-0.
Nouioui, M. A., Araoud, M., Milliand, M.-L., Bessueille-Barbier,
F., Amira, D., Ayouni-Derouiche, L., and Hedhili, A. (2018). Evaluation of
the Status and the Relationship Between Essential and Toxic Elements in the
Hair of Occupationally Exposed Workers. Environmental Monitoring Assessement,
90, 1-28. https://doi.org/10.1007/s10661-018-7088-2.
Oliveira, A. F. S., Pereira, S. F. P., Silva, T. M., Rocha, R. M.,
Costa, H. C., Silva, C. S., Nogueira, D. P., Santos, D. C., And Santos, L. P.
(2020). Evaluation and Geostatistical Study of Toxicological Risk by
Water Ingestion With Al, Ba, Fe and Pb in Communities Close to Industrial Areas
in the Brazilian Amazon. Journal Brazil Chemical Society, 31, 1492-1508. https://doi.org/10.21577/0103-5053.20200036.
Oulhote,
Y., Mergler, D., Barbeau, B., Bellinger, D. C., Bouffard, T.,and Brodeur, M. E.
(2014). Neurobehavioral Function in School-Age Children Exposed to
Manganese in Drinking Water. Environmental Health Perspectives, 122, 1-9. https://doi.org/10.1289/ehp.1307918.
Park, R. M. (2013). Neurobehavioral Deficits and Parkinsonism in
Occupations With Manganese Exposure: A Review of Methodological Issues in the Epidemiological
Literature. Safety and Health at Work, 4, 123-135. https://doi.org/10.1016/j.shaw.2013.07.003.
Pereira, S. F. P., Saraiva, A. C. F., Alencar, M. I. F., Ronan, S.
E., Alencar, W. S. A., Oliveira, G. R. F., Silva, C. S., and Miranda, R. G.
(2010). Arsenic in the Hair of the Individuals in Santana-AP-Brazil : Significance
of Residence Location. Bulletin of Environmental Contamination and Toxicology,
84, 368-37. https://doi.org/10.1007/s00128-010-9969-0.
Peña-Fernández, A., González-Muñoz, M. J., and Lobo-Bedmar, M. C.
(2016). Evaluating the Effect of Age and Area of Residence in the Metal
and Metalloid Contents in Human Hair and Urban Topsoils. Environmental Science and
Pollution Research, 23, 21299-21312. https://doi.org/10.1007/s11356-016-7352-3.
Queiroz,
T. K. L., Naka, K. S., Mendes, L. C. S., Costa, B. N. S., Jesus, I. M., Câmara,
V. M., and Lima, M. O. (2019). Human Blood Lead Levels and the First
Evidence of Environmental Exposure To Industrial Pollutants in the Amazon. International
Journal Environmental Research Public Health, 16, 1-15. https://doi.org/10.3390/ijerph16173047.
Riojas-Rodríguez,
H., Solís-Vivanco, R., Schilmann, A., Montes, S., Rodríguez, S., and Ríos, C.
(2010). Intellectual Function in Mexican Children Living in a Mining
Area and Environmentally Exposed to Manganese. Environmental Health
Perspectives, 118, 1465-1470. https://doi.org/10.1289/ehp.0901229.
Röllin, H. B., and Nogueira, C. M. C. A. (2011). Manganese: Environmental Pollution and Health Effects. Encyclopedia Environmental Health, 617-629. https://doi.org/10.1016/B978-0-444-52272-6.00540-7.
Sazakli, E., and Leotsinidis, M. (2017). Hair Biomonitoring and Health Status of a General Population Exposed to Nickel. Journal of Trace Elements in Medicine Biology, 43, 161-168. https://doi.org/10.1016/j.jtemb.2017.02.001.
Senofonte, O., Violante, N., and Caroli, S. (2000).
Assessment of Reference Values for Elements in Human Hair of Urban School Boys.
Journal Trace Elements in Medicine and Biology, 14, 6-13. https://doi.org/10.1016/S0946-672X(00)80017-6.
Sera, K., Futatsugawa, S., and Murao, S. (2002). Quantitative Analysis
of Untreated Hair Samples for Monitoring Human Exposure to Heavy Metals. Nuclear
Instruments and Methods in Physics Research Section B, 189, 174-179. https://doi.org/10.1016/S0168-583X(01)01034-5.
Skalnaya, M. G., Tinkov, A. A., Demidov, V. A., Serebryansky, E.
P., Nikonorov, A. A., and Skalny, A. V. (2016). Age-Related Differences
in Hair Trace Elements: A Cross-Sectional Study in Orenburg, Russia. Ann Hum
Biol., 43, 438-444.
https://doi.org/10.3109/03014460.2015.1071424.
Skalny, A. V., Simashkova, N. V., Skalnaya, A. A., Klyushnik, T. P., Bjørklund, G., Skalnaya, M. G., and Tinkov, A. A. (2017). Assessment of Gender and Age Effects on Serum and Hair Trace Element Levels in Children With Autism Spectrum Disorder. Metabolic Brain Disease, 32, 1675-1684. https://doi.org/10.1007/s11011-017-0056-7.
Skalny, A. V., Skalnaya, M. G., Serebryansky, E. P., Zhegalova, I. V., Grabeklis, A. R., Skalnaya, O. A., Skalnaya, A. A., Huang, P.-T., Wu, C.-C., Bykov, A. T., and Tinkov, A. A. (2018). Comparative Hair Trace Element Profile in the Population of Sakhalin and Taiwan Pacific Islands. Biological Trace Element Research, 184, 308-316. https://doi.org/10.1007/s12011-017-1204-y.
Sukumar, A. (2011). Hair for Biomonitoring of Environmental Exposures, Editor(S) : J.O. Nriagu. Encyclopedia of Environmental Health, 1-11. https://doi.org/10.1016/B978-0-444-52272-6.00370-6.
Varrica, D., Tamburo, E., Dongarrà, G., and Sposito, F. (2014).
Trace Elements in Scalp Hair of Children Chronically Exposed to Volcanic
Activity (Mt. Etna, Italy). Science of the Total Environment, 470-471, 117-126. https://doi.org/10.1016/j.scitotenv.2013.09.058.
Wang, Q., and Yang, Z. (2016). Industrial Water Pollution, Water Environment Treatment, and Health Risks in China. Environmental Pollution, 218, 358-365. http://dx.doi.org/10.1016/j.envpol.2016.07.011.
Wang, X., Ning, Y., Zhang, P., Li, C., Zhou, R., And Guo, X. (2019).
Hair Multi-Bioelement Profile of Kashin-Beck Disease in the Endemic Regions of China.
J Trace Elem Med Biol., 54, 79-97. https://doi.org/10.1016/j.jtemb.2019.04.002.
Yuen, Z., Yuzhe, W., Fanjian, M., Lifen, L., Shan, W., Xiaohui, M., Hua, L., Guixiang, Z., and Daishe, W. (2018). Distribution of Metal and Metalloid Elements in Human Scalp Hair in Taiyuan, China, Ecotoxicology and Environmental Safety, 148, 538-545. https://doi.org/10.1016/j.ecoenv.2017.10.073.
Zhuang, P., Lu, H., Li, Z., Zou, B., and McBride, M. B. (2014).
Multiple Exposure and Effects Assessment of Heavy Metals in the Population Near
Mining Area in South China. Plos One, 9, 1-11. https://doi.org/10.1371/journal.pone.0094484.
Özkara, A., and Akyl, D. (2018). Environmental
Pollution and Pollutants on the Ecosystem: A Review. Turkish Journal of Scientific Reviews,
11(2), 11-17.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2022. All Rights Reserved.