
LDPE degrading ability of Riemerella Strain isolated from the garbage dumping sites of Visakhapatnam, India
Sai Spandana Bandi 1![]() , Sridevi Veluru
2
, Sridevi Veluru
2![]() , Rishi Mallisetty 3
, Rishi Mallisetty 3![]() , Husam Talib Hamzah 4
, Husam Talib Hamzah 4![]()
![]() ,
Venkata Rao Poiba 5
,
Venkata Rao Poiba 5![]() ,
,
R. Srikanth 6![]()
1 M. Tech, Biotechnology, Department of
Chemical Engineering, A U College of Engineering (A), Andhra University,
Visakhapatnam, India
2 Professor, Department of Chemical
Engineering, A U College of Engineering (A), Andhra University, Visakhapatnam,
India
3 M. Tech, Biotechnology, Department of
Chemical Engineering, A U College of Engineering (A), Andhra University,
Visakhapatnam, India
4 Ph. D Scholar, Department of Chemical Engineering, AU College of Engineering (A), Andhra University, Visakhapatnam, India
5 Assistant Professor, Department of Chemical Engineering, College of Engineering (A), Andhra University, Visakhapatnam. Andhra Pradesh, India
6 Professor, Department
of Chemical Engineering, Anil Neerukonda Institute of
Technology and Sciences (ANITS), Sangivalasa, A.P,
India
|
|
ABSTRACT |
||
|
Polyethylene is found to accumulate in the environment, posing a major ecological threat. Affordable and environmentally friendly treatments are need of the hour to combat this plastic pollution. A study was made to isolate microorganisms from a sample of garden soil and evaluate their degrading potential. The plastic sample tested in this study were Low Density Polyethylene shopping carry bag. The growth of LDPE degrading strains was carried out in Mineral salt agar medium with LDPE as the sole carbon and energy source. Six strains were isolated. One of the strains namely A3 was found to show maximum growth rate. It was known that LDPE is resilient to biodegradation. Nevertheless, the present work shows the utilization of LDPE by A3 strain as carbon source and indicates that microbes are familiarizing towards hydrocarbons. The phenotypic fingerprint like Gen III biolog was used to actuate the substrate utilization of strain A3 and identified it as, Riemerella anatipestifer. Preliminary growth studies were carried out initially. The optimal conditions were found to be pH of 7.1, temperature of 37ᵒC, contact time of 72hrs, LDPE weight of 0.042g and inoculums volume of 3v/v. LDPE degradation was confirmed by the weight loss which was found to be 20.09% after an incubation of 35 days. As per our work we found that, garden soil is a good source of bacteria that can degrade LDPE. These results also signify the potential of novel strain Riemerella Sp. to degrade LDPE films. This manuscript will pave the way for future studies on biodegradation. |
|||
|
Received 25 September 2022 Accepted 26 October 2022 Published 14 November 2022 Corresponding Author Husam Talib Hamzah, alwatanaliraqi@gmail.com
DOI10.29121/granthaalayah.v10.i10.2022.4843 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2022 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: LDPE, Riemerella
Sp., Gen III Biolog, Biodegradation, Weight Loss Highlights ·
Low density polyethylene (LDPE) is an environmental concern due to
their high usage, and slow degradation in the environment. ·
Biodegradation is rapid and cost-effective procedure which produces
less or no harmful end products ·
Identification of bacterial
isolates that can degrade Low Density Polyethylene is the focus of this research. ·
To characterize the bacterial isolate by using novel technology like
Gen-III biolog software. ·
To optimize physiological parameters that affects the growth of LDPE
degrading strain. ·
To estimate the weight loss
percentage during 35 days of incubation. GRAPHICAL ABSTRACT
|
|||
1. INTRODUCTION
Plastics are petrochemicals which are converted into long chain polymers through a number of chemical processes Shimao (2001). Plastics are lightweight, inexpensive, and very durable. Based on their thermal properties, plastics are classified as thermoplastics and thermoset plastics. Thermoplastics are also considered as non-biodegradable plastics. Some of the Examples are Polyethylene (PE), Polypropylene, polystyrene, polyvinyl chloride, and polytetrafluoroethylenes). The most prevalent form of non-biodegradable solid waste that poses a serious risk to human life is low density polyethylene (LDPE). Fishes, birds, and mammals may sometimes get effected with intestinal blockage as a result of LDPE. LDPE degradation is a significant challenge. Each year, the consumption or entrapment of plastics in the water results in the death of nearly one million birds and 10,000 marine species World Health Organization (2011).
Some of the plastic products mimic human hormones which can cause serious health issues to human beings. The International Agency for the Research on Cancer (IARC) has determined that vinyl chloride is human carcinogenic Rudel et al., (2007). Numerous consumer products, such as toys, building materials, adhesives, detergents, lubricating oils, solvents, automotive plastics, plastic clothes, personal care items (including soap, shampoo, deodorants, scents, hair spray, nail polish), and toys all contain PVC (poly vinyl chloride) IARC (2011). In animal experiments, styrene was found to cause mammary gland tumours and was categorised by the IARC as probably human carcinogenic. It also has endocrine-disrupting effects Gray et al. (2009). Premature birth, intrauterine growth retardation, preeclampsia, and stillbirth have all been associated to BPA Benachour and Aris (2009). Additionally, it has been reported that long-term exposure to BPA has a impact on women's progesterone levels, one of the sex hormones Hao et al. (2011). Human thyroid dysfunction is caused due to phthalates and bisphenol A. Raziyafathima et al. (2016).
Around the middle of the 20th century, the majority of the plastic varieties currently in use started to be produced on a big scale. Industrial production of polyethylene (PE) began in the 1930's Andrady and Neal (2009), Howard (2002). Ever since, plastics manufacturing has been a steadily growing industry, reaching a production volume of 299 MMT (Million Metric Tonnes) in 2013 Plastics Europe (2015).
Over the past three decades, plastic materials have been employed extensively in a variety of businesses, including those that deal with transportation, food, clothes, medicine, recreation, fishing nets, packaging, and the food industry Vatseldutt and Anbuselvi (2014). A plastic object will take one of the following three pathways once it has served its purpose:
· Recycling: i.e., reuse of the material. This is considered as one of the environmentally friendly alternatives for the production of new plastic items. About 70% of all plastic has been used only once and discarded, while only about 6% of the total has been recycled. This is because each plastic has different material properties and not all the types can be recycled.
· Burning plastics to recover their energy content is another option but doing so frequently results in the release of toxic chemicals like dioxins and furans, which are harmful greenhouse gases that contribute significantly to the ozone layer's depletion. Dioxins actually cause significant issues with the activity of human endocrine hormones, which makes them a serious threat to human health Pilz et al. (2010), Chintan Environmental Organisation (2002).
· The left-over plastic waste goes to landfills, where it remains buried.
However, plastic materials are lost during their whole life cycle and end up in the environment. Despite the fact that photo degradability was employed as a remedy for plastic litter Hao et al. (2011), it has limited real-world applicability because landfills are impermeable to UV light. In order to address the issue of plastic waste, biodegradation of plastics has been suggested. Biodegradation is the term for any physical or chemical changes to a material brought on by the activity of microbes. Microbes can break down both natural and synthetic polymers Ishigaki et al. (2004).
Plastics are incredibly useful materials that have significantly altered our life in many ways. There is a massive amount of plastic pollution in both man-made and natural habitats due to its widespread use. One of the most important ways to lessen the effects of this plastic pollution is by biodegradation of plastics. But the major challenges is in taking lab-scale research into the field, understanding microbial processes at the polluted niche, and survival of non-native species and lack of integrated multi-disciplinary approach. Not only these, bioremediation process is struggling even at the laboratory scale i.e., to identify the nomenclature of biodegrading bacterial species from mixed culture of microbes, involves lot of morphological & biochemical tests and some may even take months of time to identify the name of unknown species. To overcome all these limitations, we have curated the highly sophisticated technology like GEN III, which works based on the carbon utilization of the bacterial species. Using this technology, we can identify the name of unknown species within 24hrs.
The novel GEN-III is used to identify the unknown range of Gram-negative and Gram-positive bacteria Punny et al. (2018) . GEN-III Biolog has 96 wells which works based on the cell's carbon utilization, pH, salt, and lactic acid tolerance, reducing power, and chemical sensitivity. The primary goal of this research is to look into the ability of newly isolated bacteria to degrade LDPE, with the following specific goals in mind: Isolation, screening, and identification of LDPE degrading microorganisms from soil effluent, Characterization using Gen-III biolog, and to improve the physiological parameters that influence the biodegradation of LDPE films.
2. MATERIALS AND METHODS
2.1. Sample Collection
Garden soil sample was collected from Visakhapatnam, India and placed in sterile plastic bags.
2.2. Sample Preparation
One gram of the collected soil sample was diluted in 10 ml of distilled water. The soil sample was mechanically agitated for about 10 minutes to allow the heavier particles for settling down. Upper layer of the shaken sample about 1ml was collected for further bacteriological analysis. Then 1ml of this culture is poured in a test tube containing 9ml of distilled water. It was then moved from test tube 1 to test tube 2 and so on the sample was serially diluted.
2.3. Pre-treatment of LDPE
The LDPE (low density polyethylene) shopping carry bag of
thickness 50 microns was cut in 1*1cm discs and were soaked in distilled water
for about one hour. Then they were aseptically transferred to a 70% v/v ethanol
solution for 30 minutes. The LDPE discs were then air dried and pre weighed.
2.4. Isolation of Microorganisms
capable of degradation of LDPE
Pour plate method is used to isolate microorganisms capable of degrading LDPE. MSAM (mineral salt agar medium) is poured into a Petri dish along with 1ml of diluted sample and LDPE films for carbon source. This step is repeated for each plated dilution. All the petri plates were incubated at 37°C for about a week with regular observations. After week well-defined colonies were grown on agar plates which were then further transferred to the agar slants with the same medium using the streak plate method. On MSAM agar slants, isolates were grown and sub-cultured. The isolates that grew well in MSAM were stored at 37oC and labelled A1, A2, A3, A4, A5, and A6 for future research.
2.5. GEN III BIOLOG
The GEN III biolog programme can be used to identify unknown bacterial isolates. GEN III Biolog, which was initially discovered in 1989, includes micro plates and a database. It uses a revolutionary, patented redox chemistry that is used to evaluate the genes and species and offers a clear indication for gene and species identification. It works well against a variety of gram positive and gram-negative bacteria. In GEN III, the cell's have the capacity to metabolize each major class of biochemicals together with other crucial physiological traits including pH, salt and reducing power, lactic acid tolerance, and chemical sensitivity. The test panel consists of microorganisms with a "Phenotypic Fingerprint" that can be used to identify them down to the species level. Gram-negative and Gram-positive bacteria can be tested on the same GEN III Micro-Plates panel with 71 carbon sources and 23 chemical sensitivity assays. The proprietary Tetrazolium dye breaks down as cells ingest nutrients, breathe, and expend energy, giving cells their distinctive purple colour. A unique metabolic profile (from Biolog, Inc., Hayward, USA) is entered into a computer using Biolog data collection software (Retrospect software), which may then be compared to thousands of other profiles (matching to thousands of species) kept in Biolog databases.
GEN III microbial ID assay is a one minute set up protocol. It has a database of over 1300 species. Using this methodology there is no need of gram staining or any follow-on testing. Biolog solution is setup to 100% transmittance initially, after mixing the bacterial culture from agar-streaked plate, the solution transmittance is made above 95%. Biolog data collection software (Retrospect software) is used in a computer to record a unique metabolic profile, which can then be compared to thousands of profiles stored in Biolog databases. The computer displays the species if the profile matches.
2.6. Preliminary studies to
Determine Optimal Conditions
Identified newly isolated bacterial cultures were grown overnight in nutrient broth on an orbital shaker at 120 rpm at 37°C. This 24-hour young culture was used to study the optimization of LDPE biodegradation.
To test the degradation of LDPE by newly isolated bacteria, the following parameters were optimized: Contact time (24, 48, 72, 96 & 120 hrs), LDPE film weight (0.021, 0.028, 0.035 & 0.042 gm), inoculum volume (1-4 v/v), temperature (32, 37 & 420C), and pH (5.4, 7.1 & 9.7) of MSM medium. Optimal growth conditions for effective degradation were carried out in a 100 ml of MSM (mineral salt medium) having LDPE films as the sole carbon source. To begin the cultivation and growth of LDPE degrading strain, the medium was inoculated with A3 strain. At regular intervals, samples were analyzed with a UV Spectrophotometer at 620 nm.
2.7. Dry weight determination of
recovered LDPE
Through percent weight loss, several studies in the literature have shown that bacterial isolates have the potential to degrade LDPE. Mineral salt media (MSM), along with a pure culture of Riemerella and pre-weighed LDPE films, was employed as the culture medium in this instance. It was incubated at 37° C for a month while being shaken at 125 rpm. Control was kept in the mineral salt media without culture. Each week LDPE films were removed, washed in 70% ethanol to eliminate any remaining cell mass, and then heat dried for 24 hours at 45° C. LDPE weight loss was observed. The following formula was used to calculate the amount of weight loss of the LDPE in this study:
![]()
3. RESULTS AND DISCUSSION
Most of the studies suggest bacterial species were more efficient in degrading LDPE films as compared to other isolates. It is attempted to isolate efficient LDPE degrading organisms from garden soil.
3.1. Isolation of LDPE degrading
bacteria
Bacterial strains A1, A2, A3, A4, A5, and A6 are isolated. The growth in each of these bacterial strains was evaluated. The isolated strain A3 showed the maximum growth rate using LDPE as a sole carbon source Figure 1 in sterile Mineral salt agar medium. Microorganisms have successfully adapted to given conditions and have the ability to degrade. Six isolates were chosen. A3 has grown well at a faster rate compared to the tested strains. By streak plate method on Mineral salt agar medium (MSAM) plates, pure strains capable of degrading LDPE were identified.
Figure 1
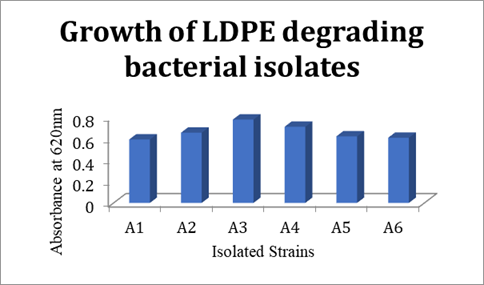
|
Figure 1 Isolated strains Vs Absorbance at 620nm |
3.2. CHARACTERIZATION OF BACTERIAL ISOLATE USING GEN III BIOLOG
The relative capacity of substrate utilization of strain A3 was tested using GENIII micro-plate. After 24 hours of incubation, the isolate reacted significantly with 59 of the 96 carbon substrates. The reaction profile was most similar to that of Riemerella strain, according to biolog data base identification Figure 2.
Systematic classification of Riemerella strain: Gram-negative bacterium that causes septicaemia and death in young ducks and geese.
Order: Flavobacteriales
Scientific name: Riemerella anatipestifer
Higher classification: Riemerella
Rank: Species
Family: Weeksellaceae
Figure 2
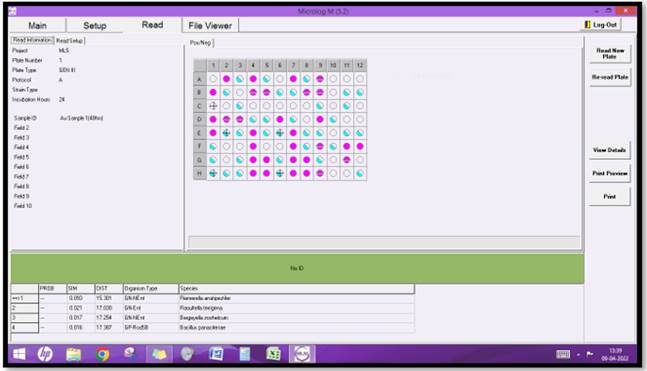
|
Figure 2 Probability Detected Isolate by Biolog Geniii Microplate in A3 |
3.3. Physiological parameters optimization (contact time, LDPE weight, temperature and pH)
The growth of any microorganism is determined by physiological parameters such as contact time, LDPE weight, inoculum volume, incubator temperature, and pH of the medium. One of the main goals of this project was to optimize these parameters. LDPE biodegradation was studied at various contact times, LDPE weights, temperatures, inoculum volumes, and pH levels.
3.3.1. Effect of Contact Time
The growth of LDPE degrading bacterial isolate by Riemerella sp. was studied over time Figure 3. It was discovered
that as contact time increased from 24 to 72 hours, the percentage growth
increased. These findings indicate that the medium's contact time is also an
important factor in degradation. After 72 hours, the rate of degradation is
constant. The investigations have been made on exposure time to the
response of pure cultures and microbial communities to LDPE toxicity.
Figure 3
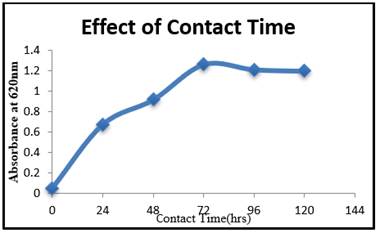
|
Figure 3 Contact Time (Hrs) Vs Absorbance at 620nm |
3.3.2. EFFECT OF LDPE WEIGHT
Experiments were carried out to investigate the effect of LDPE weight on microorganism growth Figure 4. Microorganism growth by isolated Riemerella sp. resulted in raise of absorbance after 0.042g weight. The results indicate that with increasing in the weight causes an increase in the stationary phase.
Figure 4
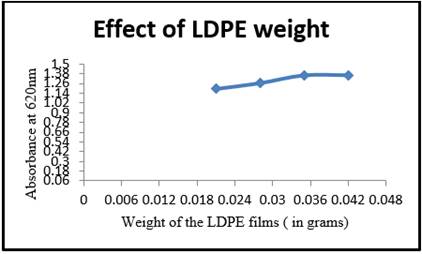
|
Figure 4 Weight of the LDPE Films (gms) Vs Absorbance at 620nm (A3) |
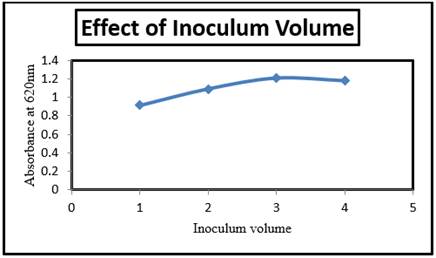
3.3.3. Effect of Inoculum volume
Microorganism growth by isolated Riemerella sp. at different inoculum volumes is depicted in the Figure 5. The absorbance value
increased from 0.917 to 1.177 with inoculum volume increase from 1v/v to 3v/v.
These results suggest that the volume of the inoculums is a significant factor
in degradation. After inoculums of volume 3v/v, the rate of absorbance tends to
decrease rapidly. The best growth growth rate for
LDPE-resistant-resistant Riemerella sp. was suggested to be 3v/v, and after
that the growth rate has been lowered.
Figure 5
|
Figure 5 Inoculum Volume (v/v) Vs Absorbance at 620nm |
3.3.4. EFFECT OF TEMPERATURE
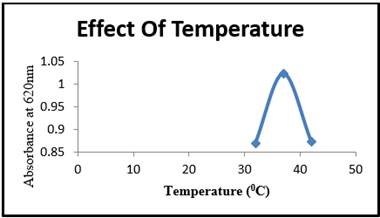
Incubator temperature has a direct relationship with microbial activity because the microbial cells responds to temperature changes through biochemical or enzymatic mechanisms. Experiments were carried out to determine the effect of temperature on cell growth over a 72-hour period at various temperatures (320 to 420C). The growth of microorganisms increased as the incubation temperature increased from 320C to 370C as shown in the Figure 6. Cells may have become metabolically active and produced enzymes required for degradation at 370c. An increase in temperature resulted in a significant reduction in the bacterial growth culture. This could have been caused by the negative effects of high temperature on enzymatic activities. It was thought that sudden exposure to temperatures above 370 could harmed the bacterial enzymes. Lower temperatures, on the other hand, could have slowed down the bacterial activity.
Figure 6
|
Figure 6 Temperature (oC) Vs Absorbance at 620nm |
3.3.5. Effect of pH
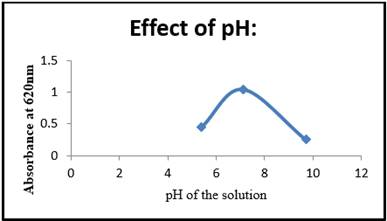
The growth of an LDPE degrading strain at various pH levels was investigated Figure 7. The growth of LDPE resistant strains was observed to increase from 0.455 to 1.040 with an increase in pH from 5.4 to 7.1 and decrease from 1.040 to 0.257 with an increase in from 7.1 to 9.7. The above results indicate that the pH of a medium is also of the important variable in bacterial growth. Bacteria showed maximum growth rate at 7.1. This research indicates that the strain actively grows at neutral of pH-7.1. Changes in pH can even cause protein denaturation, which can be harmful to the microorganism.
Figure 7
|
Figure 7 Effect of pH Vs Absorbance at 620nm |
3.4. Dry weight determination of recovered LDPE
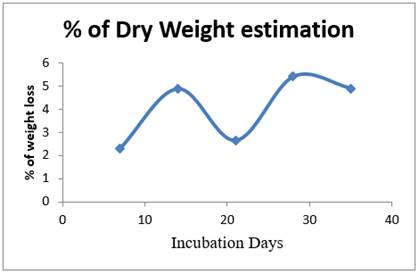
Once every seven days, LDPE strips were removed from the cultured flasks. LDPE films were then washed with distilled water and soaked for about 30 minutes to remove all the adhered cell mass from the films. The washed LDPE films were heat dried at 450c for overnight and weighed. The LDPE weight loss was calculated using percentage of LDPE degradation formula. Over 35 days, the degradation of LDPE by Riemerella sp. has been reported to be 22.09% as shown in the Figure 8 The weight loss could be attributed to Riemerella sp. consuming Low - density polyethylene as their sole carbon and energy source. In contrast, the weight of the LDPE in the control remained unchanged, as expected.
Figure 8
|
Figure 8 Incubation days Vs % of weight loss by Riemerella sp. |
4. CONCLUSION
Numerous studies have demonstrated the dramatic impact of
LDPE trash on aquatic and marine ecosystems, making it one of the biggest
environmental issues today. Physical/chemical techniques are typically used to
treat LDPE in order to eliminate such a threat.
However, processing LDPE chemically releases chemicals into the environment
along with hazardous by products. The physical approach, which is a
time-consuming and expensive process, is not preferable. In
order to produce environmentally friendly by products, we should rely on
environmental sources. The conversion of plastics into biofuel is expensive,
however by products like biofuel could be used for a sustainable environment.
Therefore, biodegradation is the more effective, economical, and sustainable
method of removing LDPE or any other contaminant. Biodegradation is an
enzymatic degradation process that involves bacteria and other microbes. This
method can be used to stop the problem of plastic waste. Several processes,
including biodegradation, depolymerization, assimilation, and mineralization,
are involved in the biodegradation of LDPE waste. Numerous enzymes secreted by
bacteria during this process will break down and transform LDPE polymers into
microbial biomass and gases. As a result, this technique has minimal or no
negative effects. The essential aspect to increase bacteria's capacity to break
down plastic waste is the optimization of appropriate environmental variables.
The study focuses on isolating, identifying, and optimising bacterial strains
from Visakhapatnam's waste dump site. Low Density Polyethylene (LDPE) is used
as the only carbon source when enriched mineral salt media is used to grow
isolated bacterial strains. All of the strains' LDPE
growth resistance capability was initially assessed. One of the strains, A3,
was discovered to be very powerful. According to the Gen III Microlog report's analysis of the isolated A3 strain, the
LDPE-degrading bacteria belong to the Riemerella
species Riemerella anatipestifer,
which has a rapid growth rate. The ideal conditions for Riemerella's
development and degradation of LDPE were discovered to be 72 hours of contact
time, 0.042 g of LDPE film weight, 3 ml of inoculum volume, 370 c,
and a pH of 7.1. According to estimates based on percentage of dry weight, Riemerella sp. might destroy 20.09 percent of LDPE over the
course of 35 days of incubation when given the best circumstances for growth.
CONFLICT OF INTERESTS
The authors declare that they do not have any known competing financial interests or personal relationships that could appear to have influenced the work reported in this paper.
ACKNOWLEDGMENTS
The authors would like to thank the Department of Chemical Engineering at Andhra University in Visakhapatnam, Andhra Pradesh, India, for providing the laboratory study for this work.
REFERENCES
Alshehrei, F., (2017). Biodegradation of Synthetic And Natural Plastic By Microorganisms. Journal of Applied and Environmental Microbiology, 5 (1), 8-19.
Andrady,
A.L., and Neal, M.A., (2009). Applications and societal benefits of
plastics. Philosophical Transactions of the Royal Society B: Biological
Sciences, 364, 1977-1984.
https://doi.org/10.1098/rstb.2008.0304.
Benachour, N., and Aris, A., (2009).
Toxic Effects of Low Doses Of Bisphenol-A on Human Placental Cells. Toxicol
Appl Pharmacol, 241(3), 322-328. https://doi.org/10.1016/j.taap.2009.09.005.
Chaturvedi, B. and Chintan., (2002). Imports Versus Surplus : A Glut of Plastics in India Today. No Plastics in the Environment (NoPE). Environmental Organisation, New Delhi.
Gray, J., Evans, N., Taylor, B., Rizzo, J.J., and Walker, M., (2009). State of the Evidence : The Connection Between Breast Cancer and the Environment. Int J Occu Environ Heal, 15(1), 43-78. https://doi.org/10.1186/s12940-017-0287-4.
Hao, J., Wang, J., Zhao, W., Ding, L., Gao, E.,Yuan, W., and Wei, S. Y. J., (2011). Effect of Bisphenol A Exposure on Sex Hormone Level In Occupational Women. 40(3), 312- 214.
Howard, G.T. (2002). Biodegradation of Polyurethane: A Review. Int Biodeterior Biodegrad. 49, 245-252. https://doi.org/10.1016/S0964-8305(02)00051-3.
Ishigaki, T., Sugano, W., Nakanishi, A., Tateda, M., Ike, M.
and Fujita, M. (2004). The
Degradability of Biodegradable Plastics in Aerobic and Anaerobic Waste Landfill
Model Reactors. 54(3), 0-233. https://doi.org/10.1016/S0045-6535(03)00750-1.
Lyon, F., (2011). Agents Classified by the IARC Monographs. World Health Organization. IARC (International Agency for The Research on Cancer), 321.
Pilz, H., Brandt, B., and Fehringer, R., (2010). The Impact of Plastics on Life Cycle Energy Consumption and Greenhouse Gas Emissions in Europe Summary Report. Vienna, Austria. Denkstatt Gmbh.
Plastics Europe., (2015). Business data and charts 2013/2014. Plastics Europe. Brussels.
Punny, K., Divya Teja, D., and Sridevi, V. (2018). A Review On Microbial Identification System-Gen III Micro-Log, A Unique Metabolic Finger-Print For Identification Of Phenol Degrading Bacteria. Int J Pharma Bio Sci, 9(2),334-339. http://dx.doi.org/10.22192/ijarbs.2020.07.06.014.
Raziyafathima, M., Praseetha, P. K., and Rimal Isaac, R. S., (2016). Microbial Degradation of Plastic Waste : A Review. Journal of Pharmaceutical, Chemical and Biological Sciences, 4(2), 231-242.
Rudel, R. A., Attfield, R.K., Schifano, J.N., and Brody, JG., (2007). Chemicals Causing Mammary Gland Tumors in Animals Signal New Directions for Epidemiology, Chemicals Testing and Risk Assessment for Breast Cancer Prevention Cancer, 109(1), 2635-2666. https://doi.org/10.1002/cncr.22653.
Shimao, M., (2001). Biodegradation of Plastics. Current Opinion in Biotechnology 2001. https://doi.org/10.1016/S0958-1669(00)00206-8
Vatseldutt, and Anbuselvi, S., (2014). Isolation and Characterization of Polythene Degrading Bacteria from Polythene Dumped Garbage. Int J Pharm. 25(2), 205-206.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2022. All Rights Reserved.