
ASSESSMENT AND IDENTIFICATION OF POLYOL BASED ON PALM OIL AND NATURAL FILLING MATERIALS FOR RENEWABLE COATING PAINT APPLICATION
Teuku Rihayat 1
![]()
![]() ,
Syafruddin 1
,
Syafruddin 1![]() , Adi Saputra
Ismy 2
, Adi Saputra
Ismy 2![]() , Nurhanifa Aidy
3
, Nurhanifa Aidy
3![]() , Nurul Izza
1
, Nurul Izza
1![]()
1 Department of Chemical Engineering, Lhokseumawe State Polytechnic, Lhokseumawe,
Aceh 24301, Indonesia
2 Department of Mechanical Engineering, Lhokseumawe State Polytechnic, Lhokseumawe,
Aceh 24301, Indonesia
3 Department of Renewable Energy Engineering, Universitas Malikussaleh, Bukit Indah, Muara Satu, Aceh 24355, Indonesia
|
|
ABSTRACT |
||
|
Chitosan/clay hybrid nanoparticles have been prepared as natural antibacterial and anticorrosion agents to enhance the protective function of polyurethane-based coating paints. Chitosan is a material with antibacterial properties because it contains acetamide group which is widely used for hygiene purposes in the medical field. Clay is a natural clay particle with a hollow structure that allows loading and release of active substances such as surfactant ions that can contribute to improving the properties of the material. The polyurethane in this study was obtained from palm oil oleic acid, which was processed into polyol, toluene diisocyanate was added to produce polyurethane. Coating paint is efficiently loaded with chitosan and clay active substances which are combined to form a hybrid composite. Based on the FTIR data of polyurethane showing the formation of a hydroxyl group on the palm oil epoxide compound, the reaction lasted for 2 hours at 60oC as evidenced by the absorption of the OH wave number which widened at 3305.99 cm-1. The value of the wavelength of the OH group in the bentonite sample before and after purification has decreased, proving that the intercalation of surfactants into the bentonite interlayer causes the hydrophilic nature of bentonite to change to hydrophobicity. The d-spacing layer of raw bentonite has a maximum reflection angle peak of 7.26o with a d-spacing value of 1.132 nm, and the d-spacing size increases to 1.631 nm at the peak of the maximum reflection angle of 4.49o after purification. The presence of absorption at 1064.71 cm-1 in shrimp shell chitosan indicates COC vibration. |
|||
|
Received 20 September 2022 Accepted 21 October 2022 Published 14 November 2022 Corresponding Author Teuku Rihayat, teukurihayat@pnl.ac.id
DOI10.29121/granthaalayah.v10.i10.2022.4833 Funding: The authors
express their gratitude and thanks to the Ministry of Education, Culture,
Research, and Technology of the Republic of Indonesia for the financial
support through the grant Number 091/SPK/D4/PPK.01.APTV/VI/2022. Copyright: © 2022 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Polyurethane, Coating Application, Palm
Oil, Bentonite, Chitosan |
|||
1. INTRODUCTION
The development of technology that continues to increase is now a benchmark for developing anti-bacterial coating materials that can be used on various equipment ranging from furniture, electronics, automotive to medical. Polyurethane as a coating material has been widely used as a coating material Salwiczek et al. (2014), Adak et al. (2019), Kong et al. (2012). Polyurethanes are a special group of polymeric materials that are fundamentally different from most other types of polymers. Polyurethanes can be incorporated into various items such as paints, liquid coatings, elastomers, insulators, elastic fibers, foams, and others. The urethane group is the main repeating unit of polyurethane, resulting from the reaction between an alcohol (OH group) and an isocyanate (-NCO group). Polyurethanes also contain other groups such as ethers, esters, urea, and some aromatic compounds Omonov et al. (2017), Doley and Dolui (2018).
Pathogenic microorganisms and the infections they cause are of great concern not only to the medical field but also to materials science. The adhesion of bacterial cells to surfaces and interfaces initiates bacterial colonization and the formation of resilient bacterial communities called biofilms, which render these surfaces and interfaces susceptible to bacterial infection Shendi et al. (2017). The way that can be done to minimize the risk of damage to the surface of the material due to bacterial adhesion is to carry out material engineering or modification. In fact, some metals or materials do have innate antibacterial properties such as gold, silver, titanium, or a mixture of them with the release of silver ions. However, other products that use other sources of ingredients will require antibacterial ingredients. Moreover, the limitations of the use of these materials are risky in several fields such as food packaging, such as silver, which causes toxic effects Ourique et al. (2017). The modern approach is the use of inorganic materials which are very important especially for the use of coating materials in the food industry and agricultural products, because of their excellent properties, such as non-toxicity, biocompatibility, renewability, biodegradability, and environmental sensitivity Chaudhari et al. (2013), Rayung et al. (2019), Acik et al. (2018). The use of these antibacterial substances also greatly contributes to the prevention of industrial economic losses caused by biofouling. due to its excellent properties, such as non-toxicity, biocompatibility, renewability, biodegradability, and environmental sensitivity Zaimahwati et al. (2014). The use of these antibacterial substances also greatly contributes to the prevention of industrial economic losses caused by biofouling. due to its excellent properties, such as non-toxicity, biocompatibility, renewability, biodegradability, and environmental sensitivity Noreen et al. (2016), Teuku et al. (2019), Back et al. (2020), Sharma et al. (2020). The use of these antibacterial substances also greatly contributes to the prevention of industrial economic losses caused by biofouling.
In addition to antibacterial properties, coatings should also be able to prevent the material from being degraded by the natural process of corrosion that occurs from chemical reactions with its environment. Corrosion is a continuous process, it can be prevented by various methods such as cathodic, anodic and barrier protection. Many types of barriers such as fillers are very small (nano). Nano was chosen over micro because it is smaller in size and accommodates a larger specific surface area. Suryani et al. (2017). The decrease in particle size favors an increase in the bar-rier and mechanical properties of the coating Wang et al. (2020), Jaganathan et al. (2019). Nanoparticles that can be used in organic coatings are SiO2 K et al. (2019), TiO2 Mekewi et al. (2017), ZnO, Al2O3, Fe2O3, CaCO3 Nacas et al. (2019) where these types are mostly found in clay Nacas et al. (2019), Yan et al. (2020).
Currently, the raw material for polyols to make polyurethane is generally derived from petroleum. The availability of petroleum began to be limited and the price fluctuated Cassales et al. (2020). For this reason, polyols can be synthesized from vegetable oils such as palm oil, which is one of the largest commodities in Indonesia. Oleic acid is the main ingredient that will form the building blocks of PU, namely polyol. The isocyanate of the TDI type (toluene diisocyanate) will be obtained from commercial materials. Clay as a great potential taken directly from the North Aceh region is widespread and has not been widely used by the surrounding community Xu et al. (2020). Chitosan is synthesized from shrimp shell waste which is wasted by the food industry around our area Ridwan et al. (2018), Cheng et al. (2019).
Hybrid nanocomposite can be defined as a combined recombination of two or more materials where one or both of them are nano-sized which will give rise to new properties that are superior to or different from the properties of the original material. The introduction of inorganic nanoparticles brings new functionality to the host polymer Amjed et al. (2020). Hybrid chitosan/clay will be made into a mixture that together synergize in increasing the protective properties of PU coating materials. As an illustration, to get the benefits of this hybrid material, the active component of chitosan will be inserted into montmorillonite which is then inserted into the polymer material as a nanofiller in various doses using a solution mixing technique Javaid et al. (2020), Teuku et al. (2018). The activation of the montmorillonite layer will be carried out using a surfactant which is effective in opening the clay layer. Changes in clay properties are the result of replacing inorganic cations in the clay with organic surfactant cations. With the inclusion of surfactants into bentonite, the d-spacing in bentonite becomes larger and allows other compatible active ingredients to be inserted into it. The incorporation of nanoparticles into the polymer matrix is difficult because the dispersion formed is unstable and settles immediately. Large agglomerates reduce the protective barrier properties of the polymer matrix Rihayat et al. (2019).
In addition, the lack of functionalization of nanoparticles and their association with polymers, results in poor coating performance. In order to achieve precise dispersion and avoid drawbacks in nanocomposite coatings, surface treatment of nanoparticles has been proposed. Large surface area of filler, even at very low concentrations can significantly change the macroscopic properties of the polymer and contribute many new characteristics to the polymer, such as increased modulus and strength, heat resistance, decreased gas permeability and flameability and more optimal antibacterial and anti-corrosion properties. Hybrid chitosan/clay will be dispersed effectively in the PU polymer matrix which produces active substances that offer better protective properties than unmixed PU Ranjani et al. (2019), Yuan et al. (2017). The resulting PU nanocomposite with chitosan/clay hybrid filler was coated on mild steel (MS) and studied for its resistance to microorganisms and corrosion. decreased gas permeability and flameability as well as more optimal antibacterial and anti-corrosion properties. Hybrid chitosan/clay will be dispersed effectively in the PU polymer matrix which produces active substances that offer better protective properties than unmixed PU Yuan et al. (2017). The resulting PU nanocomposite with chitosan/clay hybrid filler was coated on mild steel (MS) and studied for its resistance to microorganisms and corrosion. decreased gas permeability and flameability as well as more optimal antibacterial and anti-corrosion properties. Hybrid chitosan/clay will be dispersed effectively in the PU polymer matrix which produces active substances that offer better protective properties than unmixed PU Guo et al. (2020). The resulting PU nanocomposite with chitosan/clay hybrid filler was coated on mild steel (MS) and studied for its resistance to microorganisms and corrosion Zulkifli et al. (2018).
The aim of this research is to innovate petroleum-based polyurethane products to be replaced with renewable resources, namely using vegetable oil (palm oil) as a base material to be used as coating paint products for various applications ranging from the automotive, construction and property industries to the medical field. To improve the physical and chemical properties of polyurethane and polyurethane, modifications were made by adding a mixture of bentonite and chitosan hybrid fillings. Bentonite was chosen because it allows polyurethane intercalation into the bentonite layer to provide superior physical properties such as heat resistance in coating paint products. Chitosan was chosen because it has antibacterial properties that can provide physical protection for objects coated with polyurethane paint from damage caused by microorganism activity.
2. EXPERIMENTAL
2.1. MATERIALS
A set of polyurethane synthesis tools, Magnetic stirrer, Bath, Centrifuge, Petri dish, Filter paper, Analytical balance, Fourier Transform Infra-Red (FTIR) Spectrophotometer, Thermal Gravimetry Analysis (TGA), Scanning Electron Microscope (SEM), Oleic acid based on palm oil, Galsial acetic acid, Aquadest, bentonite, Shrimp Shell, HNO3, HCl, AgNO3, Commercial chitosan, Toluene diisocyanate (TDI), Cetyl trimethyl ammonium bromide (CTAB), 30% H2O2, concentrated H2SO4, Methanol, Nutrient agar.
2.2. Method
2.2.1. Synthesis Polyol of Palm Oil
The polyol synthesis process goes through two stages, namely the epoxidation and hydroxylation processes. The polyol synthesis process was carried out in a 350 ml 3 neck flask equipped with a mechanical stirrer and a cooling system. In the reactor, 30 mL of 30% H2O2 was added, 50 mL of 100% CH3COOH and 2 mL of concentrated H2SO4 were added, stirred at a speed of 200 rpm and a process temperature of 40-45 oC for 1 hour, then added 100 mL of oleic acid and stirred again at a speed of 200 rpm. and a process temperature of 40-60 oC for 5 hours. Furthermore, the product is cooled to room temperature and the oil phase is separated as epoxidized oil which is then used in the hydroxylation process.
In the hydroxylation step, 100 mL of methanol was added 50 mL of glycerin, 2 mL of concentrated H2SO4 catalyst and 5 mL of water into a 350 mL three-neck flask, heated to a temperature of 40oC. The mixture was added to the epoxidized oil solution into a 350 mL three-neck flask, stirred at 50oC for 2 hours. Then cooled to room temperature. Then it was transferred to a separatory flask and the polyol formed was separated and stored in a glass bottle. Furthermore, it was analyzed by FTIR to determine the OH group in the polyol.
2.2.2. Purification of Bentonite with Surfactant Cationic
A total of 18.2 grams of cetyl trimethyl ammonium bromide (CTAB) was dissolved with 250 ml of distilled water in a 500 ml beaker glass, this solution was then heated at 80oC for 1 hour. Separately 20 grams of bentonite dissolved with 250 ml of distilled water in a 1000 ml beaker glass. Furthermore, the bentonite solution dispersion was put into the CTAB solution and stirred for 1 hour. Strain, the bentonite continues to be washed with distilled water several times until there is no more chloride or bromide. The filtrate was tested by dripping 1M AgNO3 until no white precipitate was formed. Bentonite was put in an oven at 60 oC, then filtered using a sieve tray with a size of 100 m.
2.2.3. Preparation of Chitosan
A total of 4.25 grams of chitosan was dissolved in 100 mL of 2% glacial acetic acid while stirring at 500 rpm for 2 hours at a pH of 4.0 to obtain a chitosan suspension. Furthermore, 50 mL of 0.1 N NaOH was slowly dripped into the chitosan suspension. Furthermore, the chitosan suspension was rinsed using 150 mL of distilled water or until the pH was neutral and dried in an oven at 60oC then the chitosan was analyzed.
2.2.4. Synthesis of PU modified with blend of bentonite and chitosan
Polyol, TDI, bentonite and chitosan were mixed in a glass beaker using a 600 rpm magnetic stirrer for 1 hour. In this procedure a number of bentonite and chitosan are used, each of which is 1.2.3 percent by weight (wt%). The resulting polyurethane is then cooled at room temperature. Furthermore, the chemical structure of polyurethane, chitosan, and bentonite paints was analyzed using FTIR. The heat resistance analysis of the coating paint was analyzed using TGA and the surface shape analysis using SEM.
2.3. characterization
FT-IR is used to analyze the characteristics of polymeric materials and functional group analysis. The synthesized polyurethane sample was ground using a mortar. The sample was mixed with KBr. Infrared spectroscopy of composites obtained with KBr pellets using a Shimadzu FTIR spectrophotometer. The spectra were obtained in the mid-infrared region (4000 – 400 cm-1) at room temperature. This analysis was used to test samples of polyol, bentonite, and chitosan. X-ray diffraction (XRD) analysis of the samples was carried out using a Shimadzu XRD-7000 X-Ray Diffractometer Maxima with a Cu anode tube. XRD analysis aims to determine the crystal shape of the material. Changes in diffracted intensity are measured, recorded, and plotted against the diffraction angle. Bentonite will be tested for changes before and after purification. In addition, an analysis of the morphology of bentonite will also be carried out through a scanning electron microscope. The surface image obtained is a photograph of all the bumps, indentations, and holes on the surface.
3. result and discussion
3.1. FTIR Analysis of Polyols From Palm Oil
The use of palm oil as a raw material for the manufacture of polyols. The selection of palm oil as raw material for the manufacture of polyols is based on the availability of abundant, renewable natural sources and has a higher content of unsaturated fatty acids than saturated fatty acids. Vegetable oil derivatives such as palm oil contain functional groups such as double bonds, epoxyl, hydroxyl, ester and other groups that can be used as active groups for physical transformation and are environmentally friendly. In this study, palm oil oleic acid was used as a constituent of vegetable oil-based polyurethane.
Epoxidation oil from palm oil is obtained from the reaction between paracetic acid and oleic acid. While paracetic acid is produced from the reaction between hydrogen peroxide and glacial acetic acid with the help of concentrated sulfuric acid as a catalyst. Hydrogen peroxide is used to oxidize double bonds to epoxide groups in the epoxidation process. The results of the palm oil epoxide produced from the synthesis stage have a lighter color than the color of palm oil oleic acid. The epoxide oil formed is an intermediate compound that can react further to form a diol compound, because it has two reactive sites, namely the carbonyl group that can link glycerides with fatty acids and also the epoxide group. So, the process of polyol formation will occur when the epoxide group reacts with alcohol.
Polyols are produced from the hydroxylation process of epoxide oil obtained from the epoxidation process of palm oil oleic acid. The formation of polyols occurs when the alcohol reacts with the epoxide group. In this hydroxylation process, a mixture of methanol and glycerine is used, where the use of excess methanol can reduce the occurrence of cross linkage in the obtained polyols. In polyols, the higher the cross-linking, the more difficult it is for the solvent to penetrate, and it will be difficult to react with other compounds.
Figure 1
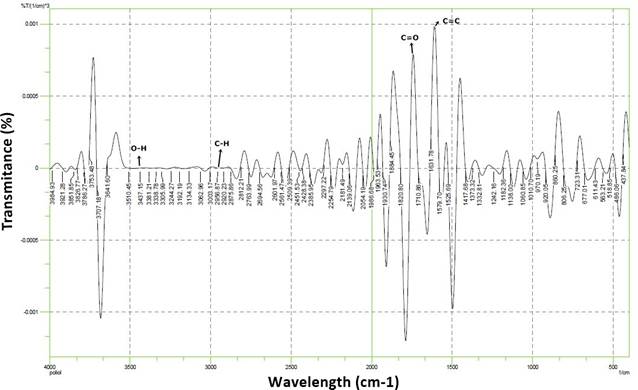
|
Figure 1 Spectrum of Ft-Ir
Polyol Oleic Acid Palm Oil |
The resulting polyol product was tested qualitatively using Fourier Transform Infra-Red (FTIR). The results of FT-IR spectroscopic analysis of polyol compounds were carried out to detect or shift peaks that could be associated with the reaction process that occurred. The spectrum of the C=O (carbonyl) group at 1680-1750 cm-1 transmission showed that the palm oil oleic acid polyol was 1710 cm-1. The presence of the C=C group is shown in the transmission of wave numbers 1550-1670 cm-1. In the polyol raw material, the wave number obtained is 1631.78 cm-1.
Polyol compounds from palm oil oleic acid that have occurred in the initial stage are the formation of epoxide intermediates through the reaction between palm oil unsaturated hydrocarbons and formic acid. The results of the FT-IR analysis have shown the formation of a hydroxyl group on the palm oil epoxide compound, the reaction lasted for 2 hours at a temperature of 60oC as evidenced by the absorption of the OH wave number which widened at 3305.99 cm-1, 3338.78 cm-1, 3381.21 cm-1 and 3450 cm-1. The hydroxy group formed is the hydroxy group on the secondary C atom. The results of the measurement of the wavelength of the hydroxyl group in previous studies were 3412.38 cm-1 Gala (2011), 3475 cm-1 Hazmi et al. (2013), 3396 Zaimahwati et al. (2015), 3384.90 cm-1 Rihayat et al. (2015).
3.2. Bentonite Characterization Using FTIR
The presence of hydroxyl groups is determined by the absorption wavelength between 3000-3600 cm-1. This states that the reacted compounds have formed the desired polyol product seen from the formation of the hydroxyl group.
FT-IR spectrum analysis shows that the bentonite sample in Figure 2. has absorption characteristics in the wave number region of 3450.9 cm-1 and 3510.33 cm-1, this is the H2O range and the OH group (hydrogen bond) which is an octahedral OH group, spectrum 1725 ,9 cm-1 bending vibration of HOH, in the spectrum 1482.3 cm-1 and 1390.94 cm-1 is OH strain.
In the FT-IR spectrum of montmorillonite processed from bentonite in Blang Dalam Village, it can be seen in Figure 4.3. The spectrum that appears is a wavelength of 3504 cm-1 which indicates the value of the OH group (hydrogen bond). The results of the measurement of the wavelength of the hydroxyl group in the previous study were 3616 to 3614 Zorica (2012) and 3424 to 3423 Wu (2014). The value of the wavelength of the OH group in the bentonite sample before and after purification has decreased, proving that the intercalation of surfactants into the bentonite interlayer causes the hydrophilic nature of bentonite to change to hydrophobicity.
Figure 2
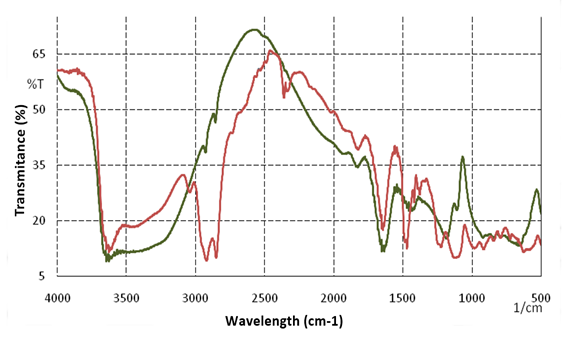
|
Figure 2 FTIR
Spectrum of Natural Bentonite (Red) And Purified Bentonite (Green) |
3.3. Morphological Characterization of Bentonite Using SEM
Scanning Electron Microscopy (SEM) is a tool used to see the morphology of a material. Based on the results of the characterization of the surface morphology with SEM, the surface structure of natural bentonite is Figure 4.4 and the purified bentonite is Figure 3.
Figure 3
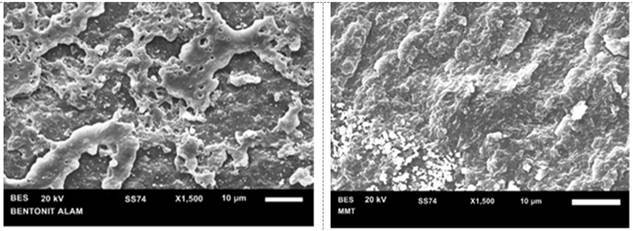
|
Figure 3 a) SEM of natural bentonite, and b) purified bentonite SEM |
Based on the results of morphological analysis of natural bentonite in Figure 3a) shows the condition of the surface of the material that is not homogeneous, there are many lumps of aggregate. However, when compared with the morphological results in Figure 3b) the shape of the surface structure of the purified bentonite has a more homogeneous morphology but there are still small aggregates in some parts.
3.4. Bentonite XRD Characterization Results
The purification process of bentonite is a step that must be carried out before bentonite is used as a filler and mixed together with polyurethane. First, the bentonite goes through the cleaning stage and the opening of the d-spacing layer. The cleaning stage aims to remove impurities from bentonite to produce pure montmorillonite. While the opening stage of the d-spacing layer with surfactants aims to increase the distance between the bentonite layers so as to maximize the interaction of the filler with the polymer.
Purified bentonite has the ability to experience swelling (swelling power) which allows the polymer to be intercalated into the mineral gallery and form nanocomposites. This swelling power will affect the quality of the bentonite produced at the time of mixing.
Figure 4
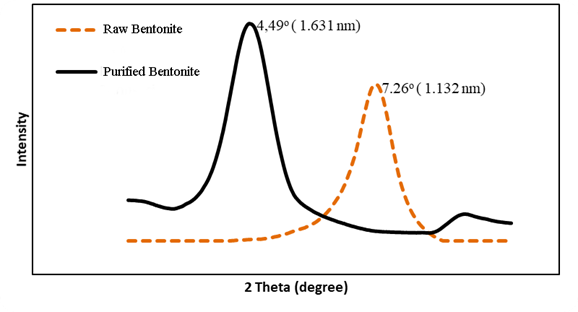
|
Figure 4 X-RD Analysis Results of Bentonite and Purified Bentonite |
X-ray diffraction (X-RD) analysis is an effective method for measuring crystal structure. If a beam of X-rays is dropped on a crystal sample, the plane of the crystal will refract X-rays that have a wavelength equal to the distance between the sides in the crystal. X-rays are refracted and captured by the detector which is then translated as a diffraction peak. This analysis aims to determine the components and the amount of minerals contained and the size of the interlayer d-spacing. The change in diffracted intensity is measured and plotted against the diffraction angle (2Ɵ). Figure 4. The following shows the results of the large test of the opening of the bentonite layer (d-spacing).
The X-RD graph plots the data between the reflection angle on the x-axis and the intensity on the y-axis to generate the bentonite d-spacing layer size automatically. By using a peak setting of 2θ which produces data at a reflection angle from 2o to 10o, based on Figure 4.6 shows the d-spacing layer of raw bentonite has a maximum reflection angle peak of 7.25o with a d-spacing value of 1.144 nm, and the size of d-spacing increases. the spacing is 1.509 nm at the peak of the maximum reflection angle of 4.50o after purification.
3.5. Chitosan Characterization Results
The analysis of the IR spectra provides information about the functional groups of the resulting products to be analysed so that it can be concluded that the compound in question has the same functional group as the expected compound. The resulting chitosan was analysed using infrared spectroscopy. The IR spectrum of chitosan is presented in Figure 5, to identify its functional groups.
Figure 5
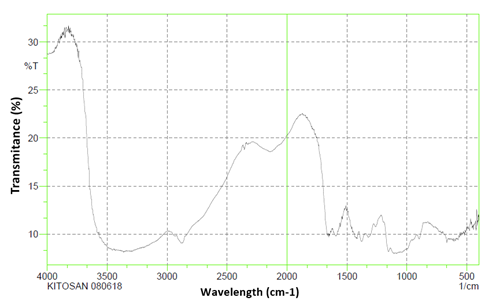
|
Figure 5 Chitosan FTIR Analysis Results |
The FT-IR spectrum analysis on the chitosan sample in Figure 4.7 shows that the chitosan spectra has an OH group absorption value at 3200 cm-1, then the presence of an NH group at 3284.77 cm-1. Another absorption is at 2879.72 cm-1 is the absorption of the methylene CH group. There is also an NH group that appears at a wave number of 1656.85 cm-1. Then there is CH3 absorption at 1425.50 cm-1. The presence of absorption at 1064.71 cm-1 on shrimp shell chitosan showed COC vibration, as well as the appearance of absorption at 668.59 cm-1 which was the NH2 group.
4. CONCLUSION
From the results of this study, it can be concluded that the synthesis of palm oil polyols can be used as a raw material for polyurethanes where there is a -OH group at a wavelength of 3524.14 cm-1 and an aliphatic CH group at a wavelength of 2805 cm-1 and a wavelength of 1054 cm-1 indicates the presence of CO groups shows that chitosan has been dispersed into a bentonite interlayer, and the isocyanate content is present at a wavelength of 1340 cm-1. The heat stability of the PU sample increases with the addition of chitosan in the PU backbone because the behaviour of chitosan is quite thermally stable but does not affect at higher concentrations. In contrast to thermal stability, anti-bacterial activity increases with increasing chitosan concentration. Finally, it was concluded that chitosan-based PU has better antibacterial and thermal characteristics for further processing, and it can be stressed that this polymer can be used in a variety of potential applications, offering unique chitosan and polyurethane characteristics. Overall, it was concluded that CS is a unique standard material for synthesis and giving extraordinary properties to PU materials.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
Acik, G., Kamaci, M., Altinkok, C., Karabulut, HRF, and Tasdelen, M. A. (2018). Synthesis and Properties of Soybean Oil-Based Biodegradable Polyurethane Films. Progress in Organic Coatings. Progress in Organic Coatings, 123, 261-266. https://doi.org/10.1016/j.porgcoat.2018.07.020.
Adak, B., Butola, B.S., and Joshi, M. (2019). Effect of Organoclay-Type and Clay-Polyurethane Interaction Chemistry for Tuning the Morphology, Gas Barrier, and Mechanical Properties of Clay/Polyurethane Nanocomposites. Applied Clay Science, 161, 343-353. https://doi.org/10.1016/j.clay.2018.04.030.
Amjed, N., Bhatti, I. A., Zia, K. M., Iqbal, J., and Jamil, Y. (2020). Synthesis and Characterization of Stable and Biological Active Chitin-Based Polyurethane Elastomers. International Journal of Biological Macromolecules, 154, 1149-1157. https://doi.org/10.1016/j.ijbiomac.2019.11.097.
Back, J.-H., Baek, D., Kim, T., Seo, B., Lee, W., & Kim, H.-J. (2020). Synthesis of Phosphorus-Containing Polyol and its Effects on Impact Resistance and Flame Retardancy of Structural Epoxy Adhesives. International Journal of Adhesion and Adhesives, 100. https://doi.org/10.1016/j.ijadhadh.2020.102601.
Cassales, A., Ramos, L.A., and Frollini, E. (2020). Synthesis of Bio-Based Polyurethanes from Kraft Lignin and Castor Oil with Simultaneous Film Formation. International Journal of Biological Macromolecules, 145, 28-41. https://doi.org/10.1016/j.ijbiomac.2019.12.173.
Chaudhari, A., Gite, V., Rajput, S., Mahulikar, P., and Kulkarni, R. (2013). Development of Eco-Friendly Polyurethane Coatings Based on Neem Oil Polyetherimide. Industrial Crops and Products, 50, 550-556. https://doi.org/10.1016/j.indcrop.2013.08.018.
Cheng, H., Liu, X., Zhang, L., Hou, B., Yu, F., Shi, Z., and Wang, X. (2019). Self-Floating Bi2s3/Poly (Vinylidene Fluoride) Composites on Polyurethane Sponges for Efficient Solar Water Purification. Solar Energy Materials and Solar Cells, 203, 110127. https://doi.org/10.1016/j.solmat.2019.110127.
Doley, S., and Dolui, S. K. (2018). Solvent and Catalyst-Free Synthesis of Sunflower Oil-Based Polyurethane Through Non-Isocyanate Route and its Coatings Properties. European Polymer Journal, 102, 161-168. https://doi.org/10.1016/j.eurpolymj.2018.03.030.
Guo, L., Pang, Z., Gao, C., Chen, X., and Liu, L. (2020). Engineering Microbial Cell Morphology and Membrane Homeostasis Toward Industrial Applications. Current Opinion in Biotechnology, 66, 18-26. https://doi.org/10.1016/j.copbio.2020.05.004.
Jaganathan, S. K., Prasath Mani, M., Ayyar, M., and Rathanasamy, R. (2019). Biomimetic Electrospun Polyurethane Matrix Composites with Tailor Made Properties for Bone Tissue Engineering Scaffolds. Polymer Testing, 78, 105955. https://doi.org/10.1016/j.polymertesting.2019.105955.
Javaid, M. A., Zia, K. M., Zafar, K., Khosa, M. K., Akram, N., Ajmal, M., Imran, M., and Iqbal, M. N. (2020). Synthesis and Molecular Characterization of Chitosan/Starch Blends- Based Polyurethanes. International Journal of Biological Macromolecules, 146, 243-252. https://doi.org/10.1016/j.ijbiomac.2019.12.234.
K., S.S., MP, I., and GR, R. (2019). Mahua Oil-Based Polyurethane/Chitosan/Nano Zno Composite Films for Biodegradable Food Packaging Applications. International Journal of Biological Macromolecules, 124, 163-174. https://doi.org/10.1016/j.ijbiomac.2018.11.195.
Kong, X., Liu, G., and Curtis, J. M. (2012). Novel Polyurethane Produced from Canola Oil-Based Poly (Ether Ester) Polyols: Synthesis, Characterization and Properties. European Polymer Journal, 48(12), 2097-2106. https://doi.org/10.1016/j.eurpolymj.2012.08.012.
Mekewi, M. A., Ramadan, A. M., ElDarse, F. M., Abdel Rehim, M.H., Mosa, N. A., and Ibrahim, M. A. (2017). Preparation and Characterization of Polyurethane Plasticizer for Flexible Packaging Applications: Natural Oils Affirmed Access. Egyptian Journal of Petroleum, 26(1), 9-15. https://doi.org/10.1016/j.ejpe.2016.02.002.
Nacas, A. M., Antonino, L. D., Chinellato, A. C., and dos Santos, D. J. (2019). Nano Boron Nitride/Polyurethane Adhesives in Flexible Laminated Food Packaging: Peeling Resistance and Permeability Properties. International Journal of Adhesion and Adhesives, 93, 102326. https://doi.org/10.1016/j.ijadhadh.2019.01.020.
Noreen, A., Zia, K. M., Zuber, M., Tabasum, S., and Zahoor, A. F. (2016). Bio-Based Polyurethane: An Efficient and Environment Friendly Coating Systems: A Review. Progress in Organic Coatings, 91, 25-32. https://doi.org/10.1016/j.porgcoat.2015.11.018.
Omonov, T. S, Kharraz, E., and Curtis, J. M. (2017). Camelina (Camelina Sativa) Oil Polyols as an Alternative to Castor Oil. Industrial Crops and Products, 107, 378-385. https://doi.org/10.1016/j.indcrop.2017.05.041.
Ourique, P. A., Krindges, I., Aguzzoli, C., Figueroa, C. A., Amalvy, J., Wanke, C. H., and Bianchi, O. (2017). Synthesis, Properties, and Applications of Hybrid Polyurethane-Urea Obtained from Air-Oxidized Soybean Oil. Progress in Organic Coatings, 108, 15-24. https://doi.org/10.1016/j.porgcoat.2017.04.002.
Ranjani, B., Pandian, K., Kumar, G. A., and Gopinath, S. C. B. (2019). D-glucosamine Chitosan Base Molecule-Assisted Synthesis of Different Shape and Sized Silver Nanoparticles by a Single Pot Method: A Greener Approach for Sensor and Microbial Applications. International Journal of Biological Macromolecules, 133, 1280-1287. https://doi.org/10.1016/j.ijbiomac.2019.04.196.
Rayung, M., Aung, Min. M., Ahmad, A., Su'ait, Mohd. S., Abdullah, L. C., and Ain Md Jamil, Siti. N. (2019). Characteristics of Ionically Conducting Jatropha Oil-Based Polyurethane Acrylate Gel Electrolyte Doped with Potassium Iodide. Materials Chemistry and Physics, 222, 110-117. https://doi.org/10.1016/j.matchemphys.2018.10.009.
Ridwan, Basuki Wirjosentono, Tamrin, R. Siburian, Teuku Rihayat, and Nurhanifa. (2018). Modification of PLA/PCL/Aceh's Bentonite Nanocomposites as Biomedical Materials. AIP Conference Proceedings, 2049(1), 02008. https://doi.org/10.1063/1.5082413.
Rihayat, Teuku, Suryani, Satriananda, Riskina, S., Syahputra, W., Nurhanifa, and Mawaddah. (2019). Formulation of Polyurethane with Bentonite-Chitosan as Filler Applied to Carbon Steel as an Antibacterial and Environmentally Friendly Paint. IOP Conf. Ser.: Mater. science. Eng, 536. https://doi.org/10.1088/1757-899x/536/1/012093.
Salwiczek, M., Qu, Y., Gardiner, J., Strugnell, R. A., Lithgow, T., McLean, K. M., and Thissen, H. (2014). Emerging Rules for Effective Antimicrobial Coatings. Trends in Biotechnology, 32(2), 82-90. https://doi.org/10.1016/j.tibtech.2013.09.008.
Sharma, S., Jain, P., and Tiwari, S. (2020). Dynamic Imine Bond-Based Chitosan Smart Hydrogel with Magnified Mechanical Strength for Controlled Drug Delivery. International Journal of Biological Macromolecules, 160, 489-495. https://doi.org/10.1016/j.ijbiomac.2020.05.221.
Shendi, H. K., Omrani, I., Ahmadi, A., Farhadian, A., Babanejad, N., and Nabid, M. R. (2017). Synthesis and Characterization of a Novel Internal Emulsifier Derived from Sunflower Oil for the Preparation of Waterborne Polyurethane and Their Application in Coatings. Progress in Organic Coatings, 105, 303-309. https://doi.org/10.1016/j.porgcoat.2016.11.033.
Suryani, Harry A., Basuki W., and Nurhanifa A. (2017). Improving the Quality of Biopolymer (Poly Lactic Acid) with the Addition of Bentonite as Filler. IOP Conference Series Materials Science and Engineering, 222(1), 012008. https://doi.org/10.1088/1757-899X/222/1/012008.
Teuku R., Suryani, Satriananda, Ridwan, Nurhanifa, Alfian P., Nia A., Muhammad Y., Sariadi, Safari, Ramzi J., Nani Siska Putri Khan, and Saifuddin. (2018). Influence of Coating Polyurethane with Mixture of Bentonite and Chitosan Nanocomposites. AIP Conference Proceedings, 2049, 1-6. https://doi.org/10.1063/1.5082425.
Teuku Rihayat, Suryani, Zaimahwati, Salmyah, Sariadi, Fitria, Satriananda, Alfian Putra, Zahra Fona, Juanda, Raudah, Aida Safitri, Mawaddah, Nurhanifa, Shafira Riskina and Wildan Syahputra. (2019). Composition on Essential Oil Extraction from Lemongrass Fragrant by Microwave Air Hydro Distillation Method to Perfume Dermatitis Production. IOP Publishing, 506(012053),1-6. https://doi.org/10.1088/1757-899X/506/1/0.
Wang, D., Zhang, N., Meng, G., He, J., and Wu, F. (2020). The Effect of Form of Carboxymethyl-Chitosan Dressings on Biological Properties in Wound Healing. Colloids and Surfaces B: Biointerfaces, 194. https://doi.org/10.1016/j.colsurfb.2020.111191.
Xu, C., Qu, Z., Tan, Z., Nan, B., Meng, H., Wu, K., Shi, J., Lu, M., and Liang, L. (2020). High-Temperature Resistance and Hydrophobic Polysiloxane-Based Polyurethane Films with Cross-Linked Structure Prepared by the Sol-Gel Process. Polymer Testing, 86. https://doi.org/10.1016/j.polymertesting.2020.106485.
Yan, J. W., Hu, C., Tong, L. H., Lei, Z. X., and Lin, Q.-B. (2020). Migration Test and Safety Assessment of Polyurethane Adhesives Used for Food-Contact Laminated Films. Food Packaging and Shelf Life, 23. https://doi.org/10.1016/j.fpsl.2019.100449.
Yuan, H., Qian, B., Chen, H., and Lan, M. (2017). The Influence of Conditioning Film on Antifouling Properties of the Polyurethane Film Modified by Chondroitin Sulfate in Urine. Applied Surface Science, 426, 587-596. https://doi.org/10.1016/j.apsusc.2017.06.314.
Yuan, H., Xue, J., Qian, B., Chen, H., Zhu, Y., and Lan, M. (2017). Preparation and Antifouling Property of Polyurethane Film Modified by Chondroitin Sulfate. Applied Surface Science, 394, 403-413. https://doi.org/10.1016/j.apsusc.2016.10.083.
Zaimahwati, Harry Agusnar, T Rihayat, D Reflianto, and S Gea. (2014). The Manufacture of Palm Oil-Based Polyurethane Nanocomposite with Organic Montmorillonite Nanoparticle as Paint Coatings. International Journal of ChemTech Research, 7, 2537-2544. http://repository.usu.ac.id/handle/123456789/71771.
Zulkifli, Teuku R., Suryani , Facraniah , Ummi H., Nia A., Teuku F., Nurhanifa , Zaimahwati, Rosalina. (2018). Purification Process of Cooking Oil Using Active Chorcoal Kepok's Banana. Aip Conference Proceedings, 1, 1-6. https://doi.org/10.1063/1.5082427.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2022. All Rights Reserved.