
Ultrasound-Assisted Adsorption Hg (II) Using Kaolin Adsorbents Modified With Anionic Surfactant
Alfian Putra 1 ![]() , Zaimahwati 1,
Rizal Syahyadi 2,
Teuku Rihayat 1, Rima Dhinta Dewi Astuti 1, Isra Adelya Izzati 1
, Zaimahwati 1,
Rizal Syahyadi 2,
Teuku Rihayat 1, Rima Dhinta Dewi Astuti 1, Isra Adelya Izzati 1
1 Department of Chemical Engineering, Lhokseumawe State Polytechnic, Jl. Medan - Banda Aceh No. Km. 280, RW. Buketrata, Punteut Mosque, Blang Mangat, Lhokseumawe City, Aceh 24301, Indonesia
2 Department of Civil Engineering, Lhokseumawe State Polytechnic, Jl. Medan - Banda Aceh No.
Km. 280, RW. Buketrata, Punteut
Mosque, Blang Mangat, Lhokseumawe
City, Aceh 24301, Indonesia
|
|
ABSTRACT |
||
|
This study aims to test the reservoir wastewater containing domestic waste using a modified kaolinite adsorbent with Alkyl Benzene Sulfonate surfactant using ultrasonic technology (KM). First, the adsorbent to be used is characterized using several different techniques such as SEM analysis, and FTIR and the calculation of the efficiency of the adsorbent concerning contact time with wastewater. Meanwhile, the wastewater tested was tested for the effect of contact time on TDS and PH. The results of the analysis show that the maximum waste reduction efficiency occurs in modified kaolin (KM), where adsorption occurs faster than in unmodified natural kaolin (PK). The maximum percentage is 84, 21% for metal removal efficiency using modified kaolin at a contact time of 45 minutes and a weight of 1.8 g of adsorbent, while kaolin without modification has an efficiency of 62.47% at a contact time of 80 minutes and a weight of 1.8 g of adsorbent. The contact time test on the TDS value of wastewater that has been adsorbed with KM shows that the TDS value is getting lower over time, which indicates the Hg (II) ion has been dispersed and fused so that the Hg (II) metal in the water is reduced. The use of the adsorption method with the help of ultrasonic technology is proven to be more efficient in accelerating the removal of Hg (II) ions by increasing the surface dispersion of the adsorbent with metal ions in water. The contact time test on the TDS value of wastewater that has been adsorbed with KM shows that the TDS value is getting lower over time, which indicates the Hg (II) ion has been dispersed and fused so that the Hg (II) metal in the water is reduced. The use of the adsorption method with the help of ultrasonic technology is proven to be more efficient in accelerating the removal of Hg (II) ions by increasing the surface dispersion of the adsorbent with metal ions in water. The contact time test on the TDS value of wastewater that has been adsorbed with KM shows that the TDS value is getting lower over time, which indicates the Hg (II) ion has been dispersed and fused so that the Hg (II) metal in the water is reduced. The use of the adsorption method with the help of ultrasonic technology is proven to be more efficient in accelerating the removal of Hg (II) ions by increasing the surface dispersion of the adsorbent with metal ions in water. |
|||
|
Received 23 September 2022 Accepted 24 October 2022 Published 14 November 2022 Corresponding Author Alfian Putra, alfianputra2021@gmail.com
DOI10.29121/granthaalayah.v10.i10.2022.4829 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2022 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Kaolinite, Adsroben,
Ultrasonic Assisted, Metal Hg, TDS |
|||
1. INTRODUCTION
A reservoir is
a place for waste disposal originating from city buildings, be it from
hospitals, government agencies, and also from households in urban areas. This
reservoir is also located in the city, so it is often a tourist destination,
but the waste that flows into this reservoir based on testing from the local
environmental service contains dangerous metals, which if these metals touch
humans will have a bad impact. for humans. The dangers of heavy metal mercury
to humans if exposed to high levels can permanently damage the brain, kidneys,
and developing fetus. Adhinugroho (2018) Effects on brain function cause
irritability, shyness, tremors, vision or hearing changes, and memory problems.
Meanwhile, short-term exposure to high levels of mercury vapor causes lung
damage, nausea, vomiting, diarrhea, increased blood pressure or heart rate,
skin rashes, and eye irritation. This waste can be handled using several
conventional methods such as chemical deposition of membranes, filtration,
coagulation, chemical extraction, ion exchange, etc. However, this conventional
technique has several disadvantages such as high energy requirements, sensitive
operating conditions, and low efficiency. Putri et al. (2022)
Figure 1

|
Figure 1 Several Types of Adsorbents Putri et al. (2022) |
To overcome
this drawback, the adsorption method is considered as one of the most
economical and efficient processes for removing harmful metal ions, due to its availability,
low cost, and operation. Estrada et al. (2016) Mahmoodi et al. (2019) Mahmoodi, N. M. (2014). This process involves separating a substance from
one phase and collecting it on another surface. This method is effective in
removing toxic pollutants, even at low concentrations, and is easy to operate. Udin (2017), Siyal et al. (2018) Adsorption is often accompanied by an
inversion-desorption process, which represents the transfer of adsorbate ions
to solution from the adsorbent surface. The reversibility of adsorption can
depend on the amount of adsorbate that is adsorbed from the adsorbent, the more
adsorbate is adsorbed, the more reversible the adsorption process. Ridwan (2018), Singh (2016) Kaolin is one of the adsorbents that is
often used in the metal ion adsorption process. Leal et al. (2017).
Kaolin (Al₂Si₂HAI(OH))
or kaolinite is an alternative material that can be used as an adsorbent.
Kaolin has a low expansion,
high chemical stability, and
cation exchange capacity. Kaolin is classified into trioctahedral and
dioctahedral minerals. Nugraha and Kulsum
(2017), Coelho et al. (2014). Kaolin has potential
as an adsorbent because it is cheap, safe, and easy to obtain, and is available worldwide in rock as a
crystalline structure. Santhosh et al. (2016), Rao
et al. (2014).
However, the absorption ability of kaolin as an adsorbent is still low when
compared to zeolite, activated charcoal., and bentonite. Mishra (2014), Efforts need to be
made to increase the adsorption ability of kaolin, namely by using surfactants
as modifying agents.
The weathering
process in the formation of kaolin occurs at or near the soil surface, which
mostly occurs in igneous rocks. Gupta et al. (2016), Emam et al. (2017). The hydrothermal alteration process occurs
because hydrothermal solution flows through fractures, faults, and other
permeable areas while converting limestone into kaolin deposits. Gupta et al. (2016), Mouni et al. (2018), Mustapha et al. (2019) Kaolin deposits consist of two kinds,
namely residues and sediments. Residual kaolin, this type is found where it
forms with the parent rock, has not undergone displacement, is crystal regular,
and ion substitution is rare. Safitri et al. (2020), Mudzielwana et al. (2019) Kaolin sediments, have been displaced by water,
wind, glaciers, deposited in basins, and irregular crystals. The structure of
kaolin can be seen in Figure 2.
The surface of
the kaolinite crystal has a constant negative charge and does not depend on pH
(permanent charge). The negative charge comes from atomic substitution in the
crystal structure which does not affect the crystal structure, for example, the
presence of an Al atom with a +3 charge replacing a Si atom with a +4 charge
causes the kaolinite skeleton to be less. positive charge or excess negative
charge. Sodeifian and Ali (2018), Roosta et al. (2017) , Suryani et al. (2020).
Figure 2
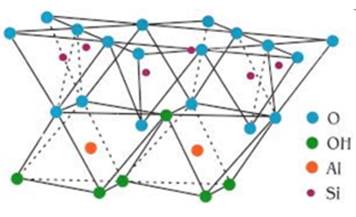
|
Figure 2 Kaolinite Structure Singh (2016) |
This
modification enhances anion absorption through ion exchange. Based on
research18, natural kaolin was successfully modified through surfactant
intercalationAlkyl Benzene Sulfonate (ABS) to the interlayers. The modified
adsorbent showed a maximum adsorption capacity of 2.3 and 2.88 m2/g. That is,
the help of ultrasonic technology can significantly improve the adsorption
ability of the adsorbent. Ultrasonic has proven to be a very useful tool in
breaking the relationship between adsorbent and adsorbate and intensifying the
mass transfer process. Raya and Zakir (2014), Jeeva et al. (2019), Wu et al. (2015).
Ultrasonic
technology has the advantages of low operating costs and no negative impact on
the environment. This technique has the effect of increasing the adsorption
capacity of the adsorbent up to two times with the help of ultrasonic speed
which in the process increases the surface area of the adsorbent.
II). Later, the wastewater to be adsorbed will be tested for TDS and PH to see
the effectiveness of the adsorbent. Suryani et al. (2018), Pakaya (2020).
2. METHODOLOGY AND CHARACTERIZATION
2.1. MATERIALS
Kaolin is taken from Nisam, North Aceh. N-Methyl-2-pyrrolidone (NMP) was
purchased from Merck and Polyethersulfone (PESf) as a binder was purchased by
Amoco Chemicals and Article 135 (CRODA). Raw kaolin and PESf were heated to 100◦C in
an oven to remove the adsorbed moisture and used without purification. Then, kaolin
was activated using 1N HCl for one hour, then washed and dried in an oven at 85
°C, sieved through a 110 mesh sieve, and then stored in a desiccator before
use. Alkyl Benzene Sulfonate (ABS) purchased from Merck
was used as a surfactant. The wastewater used in this study was taken from
the Lhokseumawe City Reservoir Waste. A sampling of the reservoir waste water
on the edge and the reservoir water has been tested to contain mercury and
several other metals. Wastewater samples were filtered using filter paper to
remove suspended solids before testing.
2.2. METHODOLOGY
2.2.1. KAOLIN SYNTHESIS WITH ABS SURFACTANT
Before being
synthesized, kaolin was activated with 1 M HCl, 120 g of activated kaolin was added
to a surfactant solution of Alkyl Benzene Sulfonate (ABS) (80 mL) and the
mixture was put into a shaker incubator at 35 oC and 150 rpm for 24 hours. The
resulting residue was washed with distilled water several times to remove
excess surfactant. The residue obtained was then dried in an oven at a
temperature of 60 oC for 12 hours and then ground using a mortar and pestle to
pass a 115 mesh sieve. KM modification used as much as 1.8 g, as well as PK,
weighed as much as 1.8 g. Each adsorbent was irradiated by ultrasonic with a
100 ml reservoir waste sample.
2.3. CHARACTERIZATION
Modified kaolin
was characterized by SEM before and after the adsorption process. The
physicochemical properties of the samples were determined by Fourier to transform
infrared spectroscopy (FT-IR), pH measurements were performed using a pH meter
every 10 minutes and TDS measurements were performed using a TDS instrument.
The ultrasonic device used is a heating system (Elmasonic, Italy) at a
frequency of 60 Hz and a power of 130 W is used for ultrasound-assisted
adsorption procedures. The adsorption of metal ions Hg(II) from water with
modified kaolin using the ultrasonic technique was studied to find the optimal
adsorbent dose, pH, TDS, and sonication time as well as the initial
concentration of Hg (II). The solution containing the metal ion Hg2+ was mixed
with natural kaolin and modified kaolin adsorbent at a certain pH (adjusted
with acetate buffer) in an ultrasonic bath for varying times. Then the
adsorbent was immediately separated from the solution and the remaining
concentration of Hg(II) ion was measured by atomic absorption
spectrophotometer. Mathematically the number of Hg (II) ions adsorbed at
equilibrium conditions (Qe, mg g-1) is obtained by using Equation 1:
Where, Co and Ce
are the initial and equilibrium concentrations of Hg (II) (mg L-1), and V and M
are the volume of solution (ml) and mass of adsorbent (g). Roosta et al. (2017).
3. RESULTS AND DISCUSSION
3.1. ADSORBENT CHARACTERIZATION
3.1.1. CHARACTERIZATION OF THE SCREENING ELECTRON MICROSCOPE (SEM)
Table 1 presents the surface area of
BET, pore diameter, and pore volume of PK and KM. The analysis revealed that after
modification with ABS surfactant, the total surface area of
kaolin clay minerals decreased drastically from 19.12 m2/g to
9.32 m2/g. On the other hand, the pore diameter and pore volume increased from
0.04 cc/g to 0.08 cc/g and 9.47 to 24.30 nm, respectively. The increase in pore
diameter and pore volume as well as a decrease in the surface area caused by surfactants that
fill most of the space on the clay surface results in an increase in pore
volume and pore diameter. The result obtained in this study is twice the
surface area of 4.3 m2/g reported by Lee et al., Rihayat et al. (2019), Safitri et al. (2020), Mudzielwana et al. (2019).
Table 1
|
Table 1 Surface Area, Pore Volume, and Pore Diameter of PK And KM |
|||
|
|
BET
surface area (m2/g) |
Pore
volume (cc/g) |
Pore
diameter(nm) |
|
PK |
19.12 |
0.04 |
9.47 |
|
KM |
9.32 |
0.08 |
24.30 |
The
morphology observed by SEM for the kaolin-modified surfactant (SMK) produced by
Ultrasonic-assisted is shown in Figure 3. It was found
that the kaolin-modified ABS surfactant was essentially ho-moddisperse
without aggregation and the nanocomposite had a cubic structure with an average
diameter of about 100 nm. KM before the Hg (II) metal adsorption process showed
that the KM surface still had many large pores Figure 3(a). Meanwhile, in
the observation of KM after the Hg (II) metal adsorption process Figure 3(b), the cavities
that have been crushed have small pores. These cavities and pores are formed
due to the absorption of Hg (II) metal in KM.
Figure 3

|
Figure 3 SEM Analysis: Kaolin (a) Before and (b) After Adsorption |
3.1.2. FOURIER TRANSFORM INFRA-RED (FTIR) CHARACTERIZATION
The
FTIR spectrum of the material was used to determine the composition and pattern
of functional groups. The FTIR spectra of natural kaolin (PK) and surfactant-modified
kaolin (KM) are presented in Figure 4. For PK, the absorption bands at 3663.23 cm-1 and 1626.17 cm-1 show the
-OH stretching vibration in water which is adsorbed by physics. The bands at
1012.50 cm-1 and 961.54 cm-1 were associated with the vibrations of the Si-OH
and Al-OH bonds. The band at 1509.46 cm-1 shows the vibration of the CC–C flex
vibration associated with the methylene group. Bands at lower wavelengths show
vibrations of Al-O-Si and Si-O-Si networks.
The spectrum of Alkyl Benzene Sulfonate shows a
stronger absorption band in the region of 2893.51 and 2944.05 cm-1, which is
bound to the CH stretching bond in the -CH3 and -CH2 groups. The bands at
925.72 cm-1 and 946.4 cm-1 correspond to CN bond vibrations. It can be seen at
the wavelengths of 2847.25 and 2975.31 cm-1 that the kaolinite adsorbent was
successfully modified which was assumed to be derived from the vibration of the
CH bond. After mercury adsorption, no changes were observed in the modified
kaolin spectrum. However, there is an increase in the transmission intensity of
the band. This could be an indication that Hg(II) can result in higher
adsorption affinity for contaminants
Figure 4
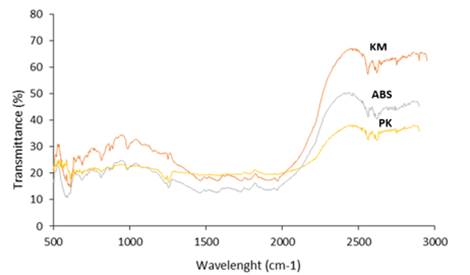
|
Figure 4 FTIR Spectrum |
3.2. EFFECT OF CONTACT TIME
3.2.1. Effect of contact time on Natural Kaolin and Modified Kaolin on Hg(II) Absorption
Batch experiments were conducted to determine the adsorption efficiency and ultrasonic-assisted adsorption process to remove Hg (II) ions in the Lhokseumawe city dam waste. The contact time required to effectively remove the metal by the adsorbent is important to determine when the equilibrium time is reached. The effect of contact time on metal adsorption on KM and PK kaolin is shown in Figure 4. The results showed a very significant difference between KM adsorbent and PK adsorbent, KM was more efficient in the adsorption of Hg (II) compared to PK adsorbent.
The results of PK
adsorption efficiency obtained the best results at a contact time of 80 minutes
at 62.74%, while the best results of KM at a contact time of 45
minutes was 84.21%. This is because Hg (II) has been adsorbed on the pores of
the KM adsorbent. After all, the molecules in the
wastewater move faster so that the interaction between the KM adsorbent and
metal ions occurs more frequently, the longer the ultrasonic contact time, the
lower the absorption efficiency due to saturation. The
adsorbent in adsorption of metal Hg(II).29
Figure 5
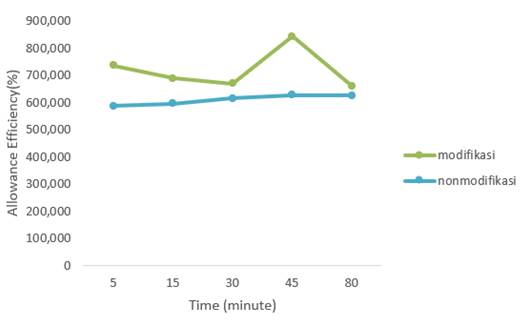
|
Figure 5 Effect of Contact Time on Hg (II) adsorption on KM and PK |
3.2.2. EFFECT OF TIME ON PH AND TDS OF WASTEWATER
The mechanism for increasing the adsorption of
kaolin adsorbents with the addition of surfactants is that the metal attaches
to the surface of the kaolin forming interactions between the molecules on the
kaolin and the surfactant. This interaction causes the formation of a new
layer, thus forming a bilayer group which results in a lot of metal ions being
adsorbed. In addition, the presence of surfactants also increases the number of
ions present on the surface of the surfactant, so that the modified adsorbent
captures more of the surrounding ions in the wastewater.
According to a study conducted by 31, adsorbed
metal Cr(IV) using anionic surfactant-modified kaolin obtained an absorption
efficiency of 95.08% at an optimal contact time of 180 minutes. the effect of
using the high-speed ultrasonic technique in the adsorption process increases
the surface area. This accelerates the movement of molecules so that the
adsorption process occurs faster.32 This phenomenon is caused by cavitation
(nucleation, growth, and collapse of small gas bubbles) and high-pressure
variations induced during ultrasonic irradiation.33
Table 2
|
Table 2 Measurement of PH and TDS |
|||
|
No |
Processing Time (minutes) |
PH |
TDS (mg/L) |
|
1 |
10 |
4.5 |
600 |
|
2 |
20 |
5 |
560 |
|
3 |
30 |
6 |
570 |
|
4 |
40 |
7 |
437 |
|
5 |
50 |
7.3 |
421 |
Figure 6
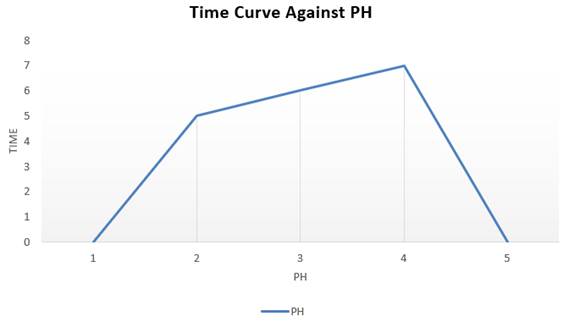
|
Figure 6 Contact Time Curve Against PH |
Figure 7
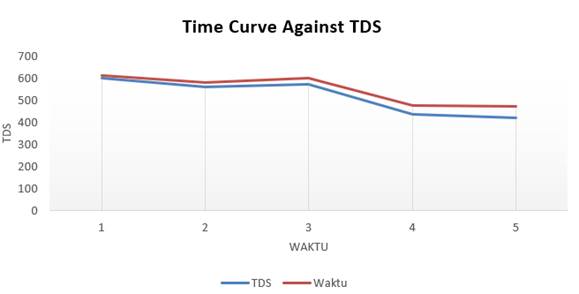
|
Figure 7 Contact Time Curve Against TDS |
The contact time of the KM adsorbent with waste also affects its PH and TDS, from Table 2 it can be seen that the PH and TDS recording time is every 10 minutes, the longer the contact time, the PH of the wastewater to its normal PH as well as the lower TDS value, this is because the longer the ultrasonic contact time, the efficiency of metal absorption decreases due to saturation of the adsorbent in adsorption of Hg(II) metal and causes the metals to be sedimented.
4. CONCLUSION
In this study, natural kaolin clay (PK) was successfully modified through intercalation of ABS surfactant to the interlayers and ultrasonic assistance to remove Hg2+ metal ions in water. The synthesized adsorbent showed the maximum adsorption efficiency of KM and PK were 84.21% and 62.74%, respectively. In this study, it was also proved that the ultrasonic adsorption method became a very useful tool in intensifying the mass transfer process and breaking the relationship between the adsorbate and the adsorbent. In comparison, ultrasonic adsorption is higher and faster than the adsorption process. Also, the efficiency of the adsorbent can be modified, which can be seen from the results of the PH and TDS testing of wastewater, which the longer the contact time the value decreases. Also, kinetic studies show that the adsorption process follows a pseudo-second order.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
Adhinugroho, P. (2018). Test the Effectiveness of Microorganisms (DDL-LActobacillus) on the Wastewater Treatment Process from Setiabudi Reservoir-PD PAL Jaya. THESIS-2000.
Anais, A., Maria, R., and Sun-Kou (2021). Comparative Study of Anion Removal Using Adsorbent Made from Homoiconic Clay. Environmental Nanotechnology, Monitoring and Management. 15. https://doi.org/10.1016/j.enmm.2021.100476.
Anirudhan, T.S., and Ramachandran, M. (2015). Removal of Basic Dye Adsorption from Aqueous Solution by Surfactant Modified Bentonite Clay (organoclay): Kinetic and Competitive Adsorption Isotherm. Process Safety and Environmental Protection, 95, 215-225. https://doi.org/10.1016/j.psep.2015.03.003.
Ayisha Sidiqua, M., and Priya, V.S. (2021). Removal of Yellow Dye Using a Composite Bound Adsorbent Developed Using Natural Clay and Activated Carbon from Sapindus Seeds. Biocatalysis and Agricultural Biotechnology, 33. https://doi.org/10.1016/j.bcab.2021.101965.
Azha, S. F., Shahadat, M., Ismail, S., Ali, S. W., Ahammad, S. Z. (2021). Prospects of Clay-Based Flexible Adsorbent Coatings as a Cleaner Production Technique Inwastewater Treatment, Challenges, and Issues : Overview. Journal of the Taiwan Institute of Chemical Engineers. 120, 178-206. https://doi.org/10.1016/j.jtice.2021.03.018.
Coelho, G.F., Goncalves JR, A.C., Tarley, C.R.T., Casarin, J., Nacke, H., and Francziskowski, M.A. (2014). Removal of Metal Ions Cd (II), Pb (II), and Cr (III) from Water by Skin Anacardium Occidental Cashew Nuts. Ecological Engineering. 73, 514 - 525. https://doi.org/10.1016/j.ecoleng.2014.09.103.
Emam, A.A., LFM Ismail, Abdel Khalek, M.A., and Rehan, A. (2017). Study of Adsorption of Several Heavy Metal Ions in Modified Kaolinite Clay. International Journal of Advances in Engineering Technology, 3, 152-163.
Estrada, J. M., and Bhamidimarri, R. (2016). A Review of the Issues and Treatment Options for Wastewater from Shale Gas Extraction by Hydraulic Fracturing. Fuel, 182, 292-303. https://doi.org/10.1016/j.fuel.2016.05.051.
Fainerman, V.B., Aksenenko, E.V., Kovalchuk, V.I., Mucic, N., Javadi, A., Liggieri, L., Schneck, E. (2020). A New View on Surfactant Adsorption at the Water/Alkane Interface - Competitive and Cooperative Effects of Surfactants and Molecules Alkane. Advances in Colloidal and Interface Science. 279. https://doi.org/10.1016/j.cis.2020.102143.
Gupta, V.K., Tyagi, I., Agarwal, S., Moradi, O., Sadegh, H., Shahryari-Ghoshekandi, R., Makhlouf, ASH, Goodarzi, M., and Garshasbi, A. (2016). Studies on Heavy Metal Ion Removal of Industrial Waste by Carbon Nanotubes : Effects of Surface Modification - A Review. Critical Reviews in Environmental Science and Technology. 46, 93- 118. https://doi.org/10.1080/10643389.2015.1061874.
Haroon, H., Ashfaq, T., Gardazi, S.M.H., Sherazi, T.A. Ali, M., Rasyid, N., Bilal, M. (2016). Kinetic and Thermodynamic Study of Cr (VI) Adsorption Equilibrium on New Adsorbent of Eucalyptus Camaldulensis Waste: Batch Reactor and Column. Korean Journal of Chemical Engineering, 33, 2898-2907. https://doi.org/10.1007/s11814-016-0160-0.
Huan, Z., Qingdong, H., Wenting, Z., Fang, G., Lei, H., Wenbo, W.
(2021). Superior Dye Removal with Recyclable Magnetic Silicate Fe3o4
Adsorbent Synthesized from Abundant Natural Mixed Clay. Chemical Engineering
Research and Design. 175, 272-282. https://doi.org/10.1016/j.cherd.2021.09.017.
Jeeva, M., Lakkaboyana, S.K., and WY, W.Z. (2019). Adsorption of Direct Brown 1 Dye Using Surfactant- Modified Kaolinite and Kaolinite. Malaysian Geological Society Bulletin, 67, 35-45. https://doi.org/10.7186/bgsm67201905.
Leal, P.V., Magriotis, Z.M., Sales, P.F., Papini, R.M., Viana, P.R. (2017). Effect of Kaolinic Acid Treatment Conditions on Ether Amine Adsorption : Comparative Analysis Using a Chamomile Tonic Tool. Journal of Environmental Management. 197, 393-403. https://doi.org/10.1016/j.jenvman.2017.04.003.
Mahmoodi, N. M. (2014). Synthesis of Core-Shell Magnetic
Adsorbent Nanoparticles and Selectivity Analysis for Binary System Dye Removal.
Journal of Industrial and Engineering Chemistry, 20(4), 2050-2058. https://doi.org/10.1016/j.jiec.2013.09.030.
Mahmoodi, N.M., Oveisi, M., Taghizadeh, A., Taghizadeh, M. (2019).
New Magnetic Amine-Activated Organic Carbon Nanotubes/Metal Nanocomposites:
from Green Ultrasound-Assisted Synthesis to Detailed Selective Pollutant
Removal Models of Binary Systems. Journal of Hazardous Materials. 368, 746-.
759. https://doi.org/10.1016/j.jhazmat.2019.01.107.
Mishra, S. P. (2014). Adsorption–desorption of heavy metal ions. Current Science, 107(4), 601–612.
Mouni, L., Belkhiri, L., Bollinger, J.C., Bouzaza, A., Assadi, A., Tirri, A., Remini, H. (2018). Removal of Methylene Blue From Aqueous Solution by Adsorption on Kaolin : Kinetic and Equilibrium Studies. Applied Clay Science. 153, 38-45. https://doi.org/10.1016/j.clay.2017.11.034.
Mudzielwana, R., Gitari, M.W., and Ndungu, P. (2019).
Performance Evaluation of Surfactant-Modified Kaolin Clay in the Adsorption of
As (III) and As(V) from Groundwater: Adsorption Kinetics, Isotherms, and
Thermodynamics. Heliyon, 5, 1-7. https://doi.org/10.1016/j.heliyon.2019.e02756.
Mustapha, S., Ndamitso, M.M., Abdulkareem, A.S., Tijani, J.O., Mohammed, A.K., and Shuaib, D.T. (2019). Potential Use of Kaolin as a Natural Adsorbent to Remove Pollutants from Tannery Wastewater. Heliyon, 5, 1-9. https://doi.org/10.1016/j.heliyon.2019.e02923.
Nugraha, I., and Kulsum, U. (2017). Synthesis and Characterization of Kaolin-ZVI (Zero Valent Iron) Composite Materials and their Application Tests as Cr (VI) Cation Adsorbents. Journal of VALENCY Chemistry, 3(1), 59-70. https://doi.org/10.15408/jkv.v3i1.4650
Ogbu, I., Akpomie, K., Osunkunle, A., and Eze, S. (2019). Sawdust-kaolinite composite as efficient sorbent for heavy metal ions. Bangladesh Journal of Scientific and Industrial Research, 54(1), 99–110. https://doi.org/10.3329/bjsir.v54i1.40736
Pakaya, K. (2020). Test Effectiveness Of Alu (Aluminum Sulfate) To Reduce Tds, Tss, And Ph Levels In Laundry Business Liquid Wastewater In Sub-District Central City, Gorontalo.Thesis,1(811415005).
Putri, H. D., Elfidasari, D., Haninah, H., and Sugoro, I. (2022). Bahaya Kandungan Logam Berat (Cd, Hg, Pb) Pada Produk Olahan Pterigoplichthys Pardalis Asal Sungai Ciliwung Jakarta Bagi Kesehatan Manusia. Jurnal Pengolahan Pangan, 7(1), 7-13. https://doi.org/10.31970/pangan.v7i1.61.
Rao, R.A.K., Ikram, S., Uddin, M.K. (2014). Removal of Cd (II) from Aqueous Solution by Exploring the\ Biosorption Characteristics of Gaozaban (Onosma Bracteatum). Journal of Environmental Chemical Engineering, 2(2), 1155-1164. https://doi.org/10.1016/j.jece.2014.04.008.
Raya, S.I., and Zakir, M. (2014). Adsorption of Pb (II) Ions on Activated Carbon from Rice Husk, Exposed to Ultrasonic Waves : Kinetic and Thermodynamic Studies. Journal of Natural Science Research. 4(2), 4-9.
Ridwan,
Wirjosentono, B., Tamrin, Siburian, R., Rihayat, T., and Nurhanifa (2018).
Modification of PLA/PCL/Aceh's Bentonite Nanocomposites as Biomedical
Materials. AIP Conference Proceedings, 2049(1), 02008. https://doi.org/10.1063/1.5082413.
Rihayat, T., and Suryani (2010). Synthesis and Properties of Biobased Polyurethane/Montmorillonite Nanocomposites. International Journal of Materials and Metallurgical Engineering, 4(5).
Rihayat, T., Hadi, A. E., Aidy, N., Safitri, A., Siregar, J. P., Cionita, T., Irawan, A. P., et al. (2021). Biodegradation of Polylactic Acid-Based Bio Composites Reinforced with Chitosan and Essential Oils as Anti-Microbial Material for Food Packaging. Polymers, 13(22), 4019. MDPI AG. http://dx.doi.org/10.3390/polym13224019.
Rihayat,
T., Suryani, Satriananda, Ridwan, Nurhanifa, Putra, A., Audina, N., Yunus, M.,
Sariadi, Safari, Jalal, R., Khan, N. S. P., and Saifuddin (2018).
Influence of Coating Polyurethane With Mixture of Bentonite and Chitosan
Nanocomposites. AIP Conference Proceedings, 2049(1), 020020. https://doi.org/10.1063/1.5082425.
Rihayat, T., Suryani, Zaimahwati, Salmyah, Sariadi, Fitria, Satriananda, Putra, A., Fona, Z., Juanda, Raudah, Safitri, A., Mawaddah, Nurhanifa, Riskina, S., and Syahputra, W., (2019). Composition of Extraction of Lemongrass Fragrant Essential Oil by Microwave Water Hydro Distillation Method on Dermatitis Perfume Production. https://doi.org/10.1088/1757-899X/506/1/012053
Roosta, M., Ghaedi, M., Shokri, N., Daneshfar, A., Sahraei, R., Asghari, A. (2017). Optimization of the Ultrasonic/Adsorption Combined Method for the Removal of Malachite Green by Gold Nanoparticles Loaded on Activated Carbon : Experimental Design. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 118, 55-65. https://doi.org/10.1016/j.saa.2013.08.082.
Safitri, N., Syahputra, R., Putri, K., Rihayat, T., Nurhanifa, A. (2020). Purifying Citronella Oil (Cymbopogon Nardus L) by Utilizing Sunlight Using Solar Cells (Photovoltaics). IOP Conference Series: Material Science and Engineering, 854, 012051. https://doi.org/10.1088/1757-899X/854/1/012051.
Saleh, A.T., Sari, A., and Tuzen, M. (2016). Chitosan-Modified Vermiculite for As(III) Adsorption from Aqueous Solution: Equilibrium, Thermodynamic and Kinetic Studies.Journal of Molecular Liquids. 219, 937-945. https://doi.org/10.1016/j.molliq.2016.03.060.
Santhosh, C., Velmurugan, V., Jacob, G., Jeong, S.K., Grace, A.N., Bhatnagar, A. (2016). The Role of Nanomaterials in Water Treatment Applications : A Review. Chemical Engineering Journal. 306, 1116 -1137. https://doi.org/10.1016/j.cej.2016.08.053.
Singh, N., and Gupta, S.K. (2016). Heavy Metal Adsorption : A Review. International Journal of Innovative Research in Science, Engineering and Technology, 5(2), 2267 -2281.
Siyal, A. A., Shamsuddin, M. R., Khan, M. I., Rabat, N. E., Zulfiqar, M., Man, Z., Siame, J., Azizlia, A. K. (2018). A Review on Geopolymers as Emerging Materials for the Adsorption of Heavy Metals and Dyes. Journal of Environmental Management, 224, 327-339. https://doi.org/10.1016/j.jenvman.2018.07.046.
Sodeifian, G., and Ali, S. (2018). Utilization of Ultrasonic-Assisted Resolv (US-RESOLV) with Polymer Stabilizers for the Production of Amiodarone Hydrochloride Nanoparticles: Process Optimization. Chemical Engineering Research and Design. 142, 268-284. https://doi.org/10.1016/j.cherd.2018.12.020.
Soltani, R. D. C., Jordi, S., Safari, M., and Rajaei, M.-S. (2016).
Sonocatalysis Enhancement of Textile Wastewater Using Bentonite-Supported ZnO
Nanoparticles: A Response Surface Methodology Approach. Journal of
Environmental Management. 179, 47-57. https://doi.org/10.1016/j.jenvman.2016.05.001.
Sun, K., Shi, Y., Chen, H., Wang, X., and Li, Z. (2015). Extending Surfactant-Modified 2:1 Clay Minerals for Absorption and Removal of Diclofenac from Water. Journal of Hazardous Materials, 323, 567-574. https://doi.org/10.1016/j.jhazmat.2016.05.038.
Suryani, Agusnar, H., Wirjosentono, B., Rihayat, T., Nurhanifa (2018). Thermal Degradation of Aceh's Bentonite Reinforced Poly Lactic Acid (PLA) Based on Renewable Resources for Packaging Application. AIP Conference Proceedings 2049, 020040. https://doi.org/10.1063/1.5082445.
Suryani, Agusnar, H., Wirjosentono, B., Rihayat, T., Nurhanifa (2018). Thermal Degradation of Acehnese Reinforced Poly Lactic Acid (PLA) Bentonite Based on Renewable Resources for Packaging Applications. AIP Conference Proceedings, 2049, 1-5. https://doi.org/10.1063/1.5082445.
Suryani, Fitria, Rihayat, Teuku, Aidy, Nurhanifa, Hasnah, T, Y. R.
(2020). Chitosan Modified Bio-Fibre Based Board as Antimicrobial and
Anti-Crack Board. IOP Conference Series: Material Science and Engineering, 854. https://doi.org/10.1088/1757-899X/854/1/012050.
Udin, M. K. (2017). Overview of Heavy Metal Adsorption by Clay
Minerals, with Special Focus on the Last Decade. Journal of Chemical
Engineering, 308, 438-62.
https://doi.org/10.1016/j.cej.2016.09.029.
Vargas, A., Cazetta, M., Kumita, T. Silva, V. Almeida (2011).
Adsorption of Methylene Blue on Activated Carbon Produced from Flamboyant Pods
{(D}elonix Regia) : A Study of Adsorption Isotherms and Kinetic Models.
Chemical Engineering Journal. 168(2), 722-730. https://doi.org/10.1016/j.cej.2011.01.067.
Wu, Q., You, R., Lv, Q., Xu, Y., You, W., Yu, Y. (2015). Efficient Simultaneous Removal of Cu (II) and Cr2O72− from Aqueous Solution by Renewable Amphoteric Functional Mesoporous Silica. Chemical Engineering Journal. 281, 491-501. https://doi.org/10.1016/j.cej.2015.07.019.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2022. All Rights Reserved.