
Introducing Methodology Detecting Sensory Sites as Emitters of Electromagnetic Radiation in WARM- AND COLD-BLOODED Animals (Introducing Distal Lizard Tails and Human Hair Follicles Commonalities)
1 BS
MBA,13442 SW 102 Lane Miami, 33186, Florida, United States
|
|
ABSTRACT |
||
|
The main
purpose of this manuscript is to introduce a simple tabletop optical
microscopy methodology allowing for the display and recording of similarities
in electromagnetic energy emission in the animal kingdom, namely the Human
Hair Follicles and Lizard’s tail tips energy emissions. This finding includes
warm- and cold-blooded specimens. Details of the technique had been developed
in 2015 and subsequently published in 2016 is presented. Since then, numerous papers were written,
they range from In Vivo experiments documenting the effect of increasing
blood alcohol levels in electromagnetic radiation in humans to the present
manuscript detailing commonalities found in human hair follicles with
spontaneous detached lizards’ tails tips. Essential the technique is the
placement of tissue in a single slide preparation (SSP) then covered by drops
of diluted Potassium Ferricyanide of formula K3[Fe (CN)6] (Figure 1). For simplicity, in this manuscript the acronyms K3Fe will
replace the formula K3[Fe (CN)6]. The intrinsic property of full absorption
of incoming electromagnetic radiation by K3Fe triggers crystallization
patterns confirming energy emissions of the tissues tested. Images,
video-recordings, and selected references to published papers are listed. |
|||
|
Received 23 August 2022 Accepted 24 September 2022 Published 12 October 2022 Corresponding Author Abraham
A. Embi, embi21@att.net
DOI10.29121/granthaalayah.v10.i9.2022.4773 Funding: This research received
no specific grant from any funding agency in the public, commercial, or
not-for-profit sectors. Copyright: © 2022 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Biophysics, Molecular, Biophysics as Bridging,
Cross Species Similarities, Potassium Ferricyanide, Absorption Incoming
Energy, Electromagnetic Energy, Cold-Warm Blooded DEFINITION OF TERMS Absorption: The transfer of the energy of a
wave to matter as the wave passes through it.... if all the energy is lost,
the medium is said to be opaque, ie: Crystallization. Anisotropy: The property of substances to
exhibit variations in physical properties along different molecular axes. It
is seen in crystals, liquid crystals and, less commonly, in liquids. Analogy
would be selecting direction of wood grain when cutting. Electromagnetic
Fields: Defined
as how matter typically electrons bound in atoms takes up a photon's energy —
and so transforms electromagnetic energy into internal energy of the
absorber. Example is the full absorption of electromagnetic radiation as
internal energy by Potassium Ferricyanide (K3Fe). Body Sensory Systems:
Essential anatomical site for EMFs emissions in animal kingdom. K3Fe: Short version for Potassium
Ferricyanide crystals with formula K3Fe (CN)6. CSA # 13746-66-2. SSP: Acronym for Single Slide
Preparation, where a plucked in toto (follicle and shaft) human hair or
animal tissue is placed on a glass slide and covered by a solution of diluted
K3Fe crystals. |
|||
1. INTRODUCTION
The introduction of a simplified method for the detection of electromagnetic energy in plants and animal tissue Benjamin et al. (2016), allowed for the recording of intrinsic similarities in organized electromagnetic energy fields (EMFs) emissions from warm and cold-blooded samples, namely distal human hair follicles and lizard’s tail tips. Details are herein presented. The intrinsic property of full absorption of incoming EMFs energy by K3Fe slows the process of crystallization patterns thus confirming the energy emissions of the tissues tested Baranov et al. (2015), Figgis et al. (1969). Still Images, video-recordings, and selected references to published papers are listed. At the end of the presentation, the reader would be able to reproduce In Vitro experiments as presented in this paper.
2. MATERIALS AND METHODS
2.1. MATERIALS
1)
Potassium FerrIcyanide Crystal.
K3Fe (CN)6.
2)
CSA # 13746-66-2.
3)
Hair Follicles plucked via tweezers from author’s
scalp, self-detached small home lizard tails.
4)
Microscope glass slides: 25x75x1mm thickness. Pearl
Cat. No. 7101
5)
Water purity confirmed by handheld electrical fields
detector manufactured by Lishtot Detection
LTD, Israel. For details link to: https://www.lishtot.com/TDP1.html
6)
Room relative humidity monitored by an ACU-RITE sensor
model # 01536-RX.
7)
Digital Video Microscope Celestron II
model # 44341, California, USA.
8)
Images downloaded to an Apple Computer MacBook Pro
Photo Application.
2.2. METHODS
2.2.1. PREPARING THE SOLUTION
Commercially available bottled
water was tested for impurities via a handheld electrical fields sensor (LishtotSensor). A solution was prepared by diluting ≅ 1 pinch of Potassium
Ferricyanide (K3Fe) crystals in 2 drops
of the previously tested for impurities bottled spring water. The solution
withdrawn as needed via pipette.
2.2.2. THE SINGLE SIDE PREPARATION (SSP)
The SSP is an open-air technique
where freshly plucked in toto human hairs or any other tissue are placed on a clean
25x75x1mm glass slide; and covered by drops of K3Fe in solution;
the liquid was then allowed to evaporate. Prior to evaporation, the drops were
gently touched by a wooden toothpick and dispersed as to cover the follicle and
shaft (see below). After the hair sample stops drifting and stabilizes, a clean
wooden toothpick was used to gently shepherd the hair sample away from the drop
edges. As evaporation starts, images and video recordings are made and stored. Figure 1
Figure 1
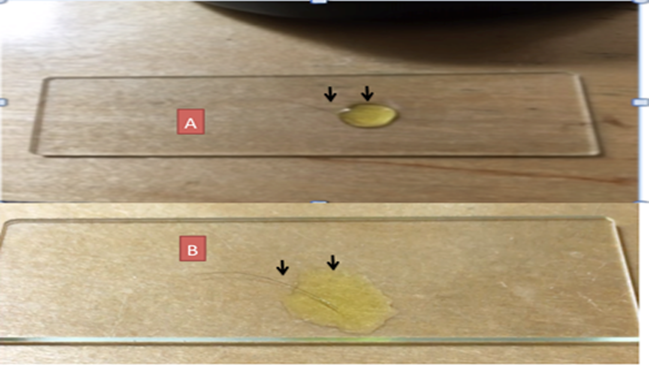
|
Figure 1 A: Scalp hair on glass slide covered by drop of (Potassium Ferricyanide) covering mainly the hair follicle. B: Same hair. Now the K3Fe drop surface tension disturbed via wooden toothpick now covering follicle and shaft. Image reproduced from: Article Citation: Embi (2022). Introducing Crystallization Backward Suction Trapping Lipids and
Debris as Proposed Additional Factor in the Genesis of Coronary Artery
Disease. International Journal of Research -Granthaalayah, 8(9), 215-233. https://doi.org/10.29121/granthaalayah.v8.i9.2020.1174 |
Post Data Processing:
Images and video recordings were
labelled, recorded, and printed for further analysis.
3. RESULTS
Freshly plucked via tweezers obtained
of author’s In Toto (hair Follicle and shaft) placed on glass slide
imbedded in liquid K3Fe. The image below shows a normal human hair post testing
Figure 2. Images of non-disturbed samples show concentric semi-circular K3Fe
crystallization images Figure 2, Figure 3)
Conversely, when both tissues
(hair follicle and Lizard tail) are cut, the cut end shows aberrant EMFs (Figure 3, Figure 4).
4. HUMAN TISSUE
Freshly Plucked Human Tissue
Samples in SSP K3Fe
Figure 2

|
Figure 2 Plucked Scalp Human Hair in SSP Imbedded in Liquid K3Fe Post Evaporation. Black Arrow: Pointing at Semi-circular Concentric Crystals Reflecting the Hair Follicle’s Electromagnetic Energy. |
5. REPTILE TISSUE
The freshly plucked tail tip
showing similar concentric precipitated K3Fe crystals as seen in Figure
2 above.
Freshly Detached Lizard Tail
Figure 3
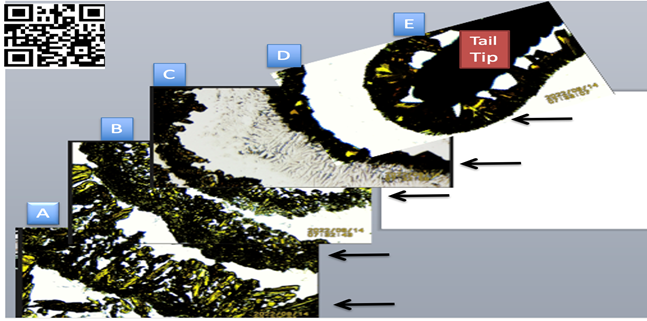
|
Figure 3 Small freshly cut lizard distal tail tip in SSP K3Fe showing increasing crystals adhesion when nearing the EMFs source. A, B,
C: Further away from tip- Notice greater dispersion of crystals
distribution. D, E: Near the tail tip
(EMF source). Notice crystals compactness. Image reproduced
from (APA): Embi, A. A. (2022). Introducing
Methodology to Detect Dead Tissue Stored Energy. International Journal of
Research-Granthaalayah, 10(8), 20–29. https://10.29121/granthaalayah.
v10.i8.2022.4733 |
6. TRANSECTED HUMAN TISSUE
Opposite
End of Cut Human Hair Follicle Tissue
Hair
Follicle
Figure 4

|
Figure 4 Large arrow: Transected hair follicle. Small top arrow: Towards Distal Follicle. X: Disorganized K3Fe crystals. Please compare with organized semicircular crystals in distal follicle end Figure 2 above. |
7. TRANSECTED REPTILE TISSUE
Opposite End of Lizard Tail Tip.
The same procedure (SSP) was
repeated, this time transected lizard tails tips were
analysed Figure 5.
Transected Tail Tip
End
Figure 5

|
Figure 5 Large arrow: Disorganized K3Fe crystallization, confirming a disturb emission of incoming electromagnetic radiation. Compare with Figure 3 above. Pointing at cut mid lizard tail point. Notice
Towards Distal Follicle. X: Disorganized K3Fe crystals. Please compare with
organized semi-circular crystals in distal follicle end Figure 2 above. |
8. DISCUSSION
The emission of EMFs by warm blooded hair follicles and
cold-blooded lizard tails are introduced. There is commonality present in both
groups, namely at the end of untouched lizard tails and hair follicles there
are organized semi-circular precipitated K3Fe crystals, indicating stable EMFs
energy emission. It must be mentioned that in the original paper introducing
the methodology Benjamin et al. (2016) the authors stated
that the precipitation of K3Fe crystals were triggered by the sensing of
undisturbed incoming EMFs. Stated was at the time “Results: As a result of their intrinsic electron transport-based metabolism these
biologic entities emitted electromagnetic fields that were imaged by aggregated
iron particles outlining the leaves or visualized as circulating aggregated
iron particles around the hair follicles. Conclusions: This technique can provide a
simplified imaging method to provide electromagnetic profiles for living
systems in general.”
Additional
Clarification
Sensory
organs are essential for the emission of EMFs in the Animal Kingdom.
Warm- and
Cold-Blooded Metabolism
The recording
of detectable electromagnetic radiation in a cold-blooded animal tail tip by
the K3Fe method was unexpected. This issue was clarified in two publications,
where the presence of spinal columns and nerves in the lizard’s tails are described,
as published “Animals signals must be detected by receiver sensory systems and
overcome a variety of local ecological factors that could otherwise affect
their transmission and reception. The lizard tail has been hypothesized be part
of the animal sensory system, since the lizard tail includes a spinal column
and nerves.”
Duffy et al. (1991), Peters and Ramos (2022).
Suggested is to repeat experiments placing the
reptile in different temperature settings. In this manuscript the lizard was
found inside an air-conditioned cooled home with 55% humidity and thermostat
set at 76 degrees Fahrenheit.
9. CONCLUSION
1) The finding of
undisturbed cold-blooded tissue
expressing electromagnetic radiation energy needs further studies at different environmental conditions. The presence of
sensory systems in an animal is reported to be essential for the emission of
electromagnetic energy.
2) The human hair follicle and lizard’s tails are confirmed
sensory systems.
3) Body temperature to be a factor.
10. The questions arise
What is the lowest body
temperature where EMFs emissions ceases?
Unknown at present
In reptiles, there appears to be
a body area (distal tail tip) where undisturbed EMFs emissions are detected. In
humans the distal hair follicle is also identified as a similar area for
organized EMFs detection.
Other Implications
The Potassium Ferricyanide
method could be used in drug evaluations or forensics by detecting EMFs signals
present in the living or the dead Embi (2022). For example, the human hair follicle has shown a marked in vivo temporary
increase in EMFs emissions post alcohol ingestion Embí (2020). Hours post consumption normal emissions returned. The findings herein
presented could expand human drug evaluations research in cold blooded animals.
11. SUPPLEMENTAL IMAGES
11.1. SUPPORTING VALIDITY OF TECHNIQUE
The validity of the K3Fe methodology was confirmed by this
non-drinker author in experiments where x2 alcohol was consumed to the point of
exhibiting typical drunken feelings. My hair follicles were analysed control
and post alcohol intake Embí (2020). I have taken the
liberty to share images from effect of 2 binge drinking episodes on my hair
follicles electromagnetic energy emissions Please see below). All 2 figures below
reproduced from reference Embí (2020) link: https://doi.org/10.29121/granthaalayah.v8.i10.2020.1568
In addition, the K3Fe method was
further confirmed in a recent paper Embi (2022).
11.2. SUPPORTING VALIDITY OF K3FE METHODOLOGY
Figure 6

|
Figure 6 Human Hair in SSP K3Fe After Evaporation, Showing: F= Follicle. Black Arrows: Pointing at Organized Concentric K3fe Crystallization Due Full Absorption of The Follicle’s EMR |
Figure 7

|
Figure 7 n=1 Showing 45 post binge episode of ingesting 250 ml of white wine, plus one 12 OZ Lager beer consumed within 15 minutes. Black Arrows: Altered organized Crystals semicircles- Indicative of electromagnetic Radiation emission disruption by BAC F: follicle. Black Arrows: K3Fe Crystals Full absorption of follicle EMRs. |
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
Benjamin J. S., Sahoo, K., and Embi, A. A. (2016). Novel and Simplified Method for Imaging the Electromagnetic Energy in Plant and Animal Tissues. Journal of Nanoscience and Nano engineering, 2 (1), 6-9.
Baranov, D. G., Edgar, J. H., Hoffman, T.,
Bassim,
N. and Caldwell, J. D. (2015). Perfect Interferenceless
Absorption at Infrared Frequencies by A Van Der Waals Crystal. Physical Review
B, 92 (20). https://doi.org/10.1103/PhysRevB.92.201405.
Duffy, M.T., Simpson, S.B. Jr., Liebich, D.R., and Davis, B.M., (1991). Origin of Spinal Cord Axons in the Lizard Regenerated Tail : Supernormal Projections from Local Spinal Neurons, J Comp Neurol, 293(2), 208-22. https://doi.org/10.1002/cne.902930205.
Embi, A. A. (2022). Introducing Methodology to Detect Dead Tissue Stored Energy. International Journal of Research - Granthaalayah, 10(8), 20–29. https://doi.org/10.29121/granthaalayah.v10.i9.2022.
Embi, A.A. (2022). Spontaneous Levitation of Plucked Human Hairs on a Glass Slide When Immersed in Liquid Potassium Ferricyanide, Supporting a Tabletop Microscopy Methodology for the Imaging of Electromagnetic Energy in Plant and Animal Tissue, International Journal of Research - Granthaalayah, 10(7), 106–111. https://doi.org/10.29121/granthaalayah.v10.i9.2022.
Embí, A. A. (2020). The Drunken Hair : Introducing in Vivo Demonstration of Increased Blood Alcohol Concentration Temporary Disrupting Human Hair Follicles Emission of Electromagnetic Radiation. International Journal of Research -Granthaalayah, 8(10), 123-130. https://doi.org/10.29121/granthaalayah.v8.i10.2020.1568.
Figgis, B. N., Gerloch, M., Mason, R. and Nyholm, R. S. (1969). The Crystallography and Paramagnetic Anisotropy of Potassium Ferricyanide. https://doi.org/10.1098/rspa.1969.0031.
Peters, R.A., Ramos, J. (2022). Properties Of An Attention-Grabbing Motion Signal : A Comparison of Tail and Body Movements in a Lizard. J Comp Physiol A 208, 373–385. https://doi.org/10.1007/s00359-022-01544-3.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2022. All Rights Reserved.