
INTRODUCING METHODOLOGY TO DETECT DEAD TISSUE STORED ENERGY
1 BS MBA,13442 SW 102 Lane Miami, 33186,
Florida, United States
|
|
ABSTRACT |
||
|
The presence of Catalase essential for dead or alive biological tissue energy emission. In a seminal
paper describing the origin of magnetic fields in the human body Cohen D. by
using sophisticated equipment stated: “Most of the field over the head is
produced by electrical sources associated with the hair follicles of the scalp; this
field is produced only as a response to touching or pressing the scalp…”. Recently, a tabletop optical microscopy
(TTM) method was developed in 2015 and published a year later by Scherlag et al. also enabling detection of
electromagnetic fields (EMFs) in plant and animal tissue. That novel
microscopy method was achieved in the absence of mechanical instrumentation
(as used by Cohen) due to a most interesting property of Potassium
Ferricyanide of formula K₃ [Fe (CN)₆ allowing for the total absorption
of incoming EMFs. For simplicity, in this manuscript K₃ [Fe (CN)₆
will be replaced by the acronym K3Fe. This manuscript applies TTM methodology
able to detect and display EMFs energy emitted from three lizards’ tails; one
estimated dead for four weeks, the second and third harvested while alive and
used as control. This communication supports a long-standing definition of
dead matter “matter composed of organic compounds that has come from the
remains of organisms such as plants and animals and their waste products in
the environment”. Experiments
herein presented support adding the presence of stored remnant energy to the
definition. This energy could now be easily displayed even in the absence of
life. |
|||
|
Received 13 July 2022 Accepted 15 August 2022 Published 27 August 2022 Corresponding Author Abraham
A. Embi, Embi21@att.net DOI 10.29121/granthaalayah.v10.i8.2022.4733 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2022 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Dead Tissue Energy, Dead Matter,
Electromagnetic Radiation, Tabletop Microscopy Method, Potassium
Ferricyanide, Catalase, Lizard Tails, Absorption Incoming Radiation Definitions of Terms Dead Tissue: Harvested samples from lizards dying of natural
causes. Dead Matter: Matter composed of organic compounds that has come
from the remains of organisms such as plants and animals and their waste
products in the environment. Dead Tissue Energy: Displayed as precipitated K3Fe crystals due to
incoming EMFs absorbed by K3Fe. Death by Natural Cause: Death occurring in the absence of external causes. EMFs: Electromagnetic Fields. K3Fe: Acronym for Potassium Ferricyanide of Formula K₃
[Fe (CN)₆. As note of interest, K3Fe has been found to fully absorb
incoming EMFs. SSP: Single slide preparation where liquid Potassium
Ferricyanide (K3Fe) drops placed on single glass slide. Sample placed on
slide. EMFs detected by K3Fe evaporation patterns. |
|||
1. INTRODUCTION
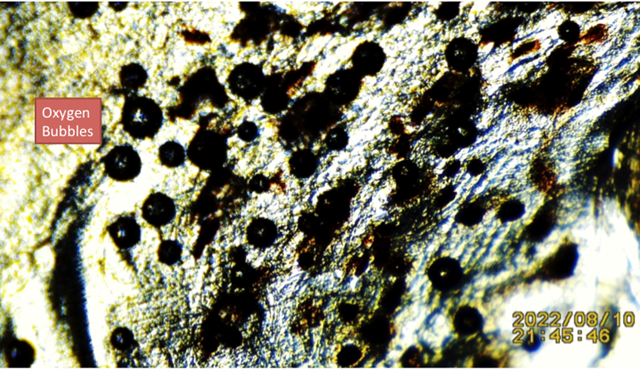
The main purpose of this manuscript is to introduce the presence of energy emitted by dead tissue harvested from three small reptile’s tails (lizards). A glass slide optical microscopy method is used to examine dead and living lizard tails tissue. The first tail found attached to a skeleton that was adhered for approximately four weeks to an outer cement wall by a rear limb (no exposure to soil); the second and third obtained after forced detachment during manual trapping and then released, used as controls (Exhibit 1). All samples were tested for the presence of the ubiquitous enzyme catalase present in living tissue; all testing positive for catalase (including the dead) by adding 3% hydrogen peroxide (H2O2) drops, oxygen bubbles were detected as shown Figure 1, Figure 2.
Exhibit 1

|
Exhibit 1 Showing A, B: Dead Lizard Leg Limb Adhered to Outer Wall for Approximately One Month. Specimen Was Detached and Tested for The Presence of Catalase. C: Second Fresh Lizard Tail D: Third Fresh Lizard Tail |
2. VIDEO-FRAME SHOWING VIGOROUS O2 BUBBLES FORMING POST ADDITION OF 3% H2O2 TO FRESHLY OBTAINED TAIL FRAGMENT
Figure 1

|
Figure 1 H2O2 Drop on Fresh Living Lizard Tissue Tail- Vigorous Oxygen Bubbles Supporting Presence Catalase For details link to: https://youtu.be/2t0QBs---JI Or Scan QR Code in Left Lower Corner |
3. MONTH OLD LIZARD SKELETON TISSUE FRAGMENT IN LIQUID SHOWING REMMANT OF MAGNETIC ATTACTION
Figure 2
|
Figure 2 Month-Old Lizard Skeleton Tissue Fragment Showing Remmants of Magnetic Attraction. Please Scan QR Code for Details |
4. MONTH OLD LIZARD SKELETON TISSUE TESTING POSITIVE FOR CATALASE
Figure 3
|
Figure 3 Showing Less Vigorous Oxygen Bubbles Post Drops Of 3% H2O2 On Dead Dehydrated Lizard Tissue (Shown in Figure 3) Exposed to Weather/Sun Exposure For 4 Weeks. Bubbles Supporting the Presence Catalase |
5. MATERIALS AND METHODS
5.1. Materials
1) Glass slides 25x75x1 mm
2) Video microscope
3) Catalase powder
4) Wooden toothpicks
5) Small transfer pipette
6) Demineralized water
5.2. Methods
Tissue sample fragments from one long-term dead lizard tail analysed. Two additional freshly (approximately 4 hours) detached lizard’s tails were secured and studied.
Using a wooden toothpick on a clean glass slide approximately a pinch (50 small crystals of K3Fe) was placed. Two drops of demineralized bottled water placed on crystals; then gently mixed with same toothpick until all crystals have dissolved. The solution is then evenly spread on the slide, and a small fragment of tissue placed in the center. As the fluid evaporates crystallization ensues, video and still images recorded for further analysis.
6. PLUCKED HAIR FOLLICLE .EXAMPLE OF K3Fe TOTALLY ABSORBING INCOMING EMFs. NOTICE COHESIVE CRYSTALS
Figure 4
|
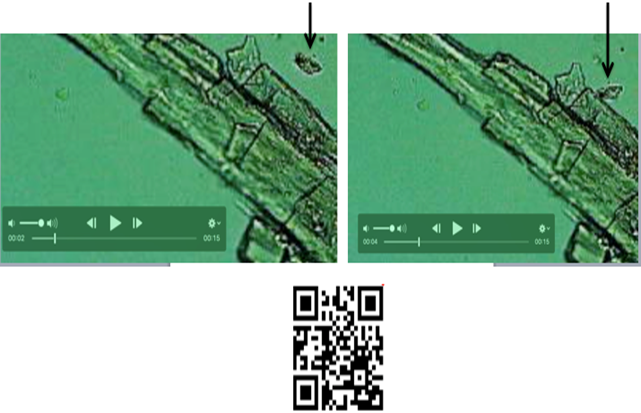
Figure 4 Black Arrow: Precipitated Crystals of Potassium Ferricyanide by Incoming Electromagnetic Radiation from A Human Hair Follicle, (Image from Hair Follicles Files) |
7. RESULTS
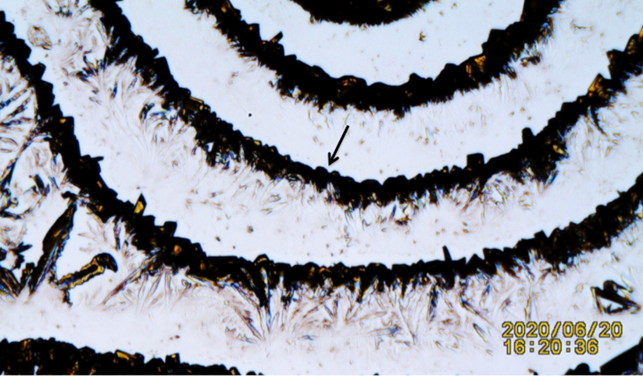
Both dead and alive lizards tissue tails when tested by TTM and shown to exhibit emission of electromagnetic energy. Two fresh tail living samples used as control. The one-month dead lizard skeleton tail displaying what appears to be less dense crystals Figure 5 theorized as result of a weaker EMFs signals. The more recent detached tissue energy sensed by the K3Fe showing a more defined and dense semi-circular crystallization images Figure 6.
NOTE: Supporting additional testing shown demonstrating weaker EMFs triggering weaker Potassium Ferricyanide crystals depositions.
Figure 5
|
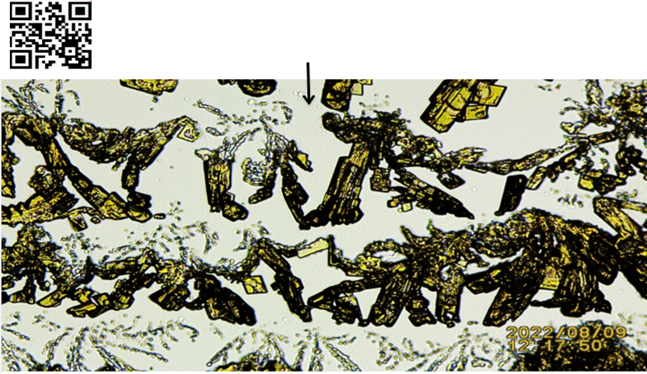
Figure 5 Amplified K3Fe Crystals Precipitated from Dead Tissue Emfs. Black Arrow: Notice Space (Less Cohesive) Between Crystals Formation For Additional Details, Please Link To: https://youtu.be/0EXN0iBs-18 Or Scan QR Code in Left Upper Corner
of Image Recommended to Fast Forward Video to Frame 03’ 55”
To Fully Appreciate Effect of Weaker Emfs on Precipitating K3Fe Crystals |
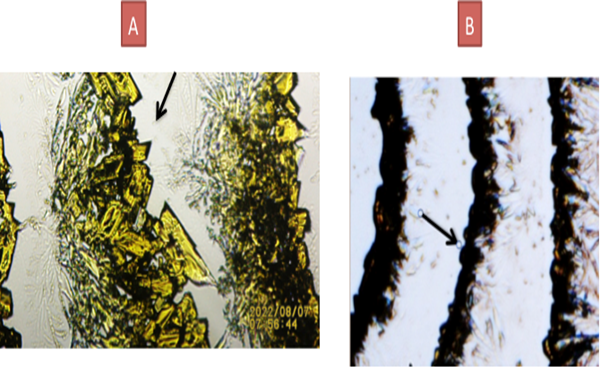
8. SELECTED IMAGES SHOWING MARKED DIFFERENCE BETWEEN CRYSTALIZATION PATTERNS BETWEEN: A: WEAK EMFs and B: STRONGER EMFs
Figure 6
|
Figure 6 A: Black Arrow. One month old dead lizard tail triggering less dense crystals. B: Black Arrow: Tail fragment from four hours post
detachment showing a more cohesive-compact crystals formation. |
9. ADDITIONAL TEST DONE ON A CUT FRESH DISTAL TAIL FRAGMENT SHOWING STRONGER EMFs CLOSER TO SOURCE TRIGGERING COHESIVE CRYSTALS.
Figure 8

|
Figure 7 Sample 3 in Exhibit 1. Small Lizard Distal Tail Tissue in SSP K3Fe Showing Potassium Ferricyanide Crystals Totally Absorbing Emfs. Notice The Further Away from The Emfs Source (Tail Fragment) The Less Cohesiveness or Compactness of The Crystals. Black Arrow: Pointing at K3Fe Crystals. For Details Link To https://youtu.be/Q-yNH7sc3m8 Or Scan QR Code in top
left of image |
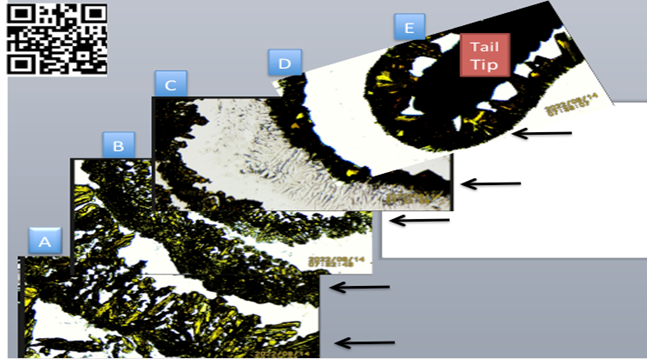
10. CUT-PASTE VIDEO FRAMES EXPANDED IMAGE OF FIGURE 5
Figure 8
|
Figure 8 Small freshly cut lizard distal tail tip in SSP K3Fe showing increasing crystals adhesion when nearing the EMFs source. A, B, C: Further away from tip- Notice greater
dispersion of crystals distribution.
D, E: Near the tail tip (EMF source). Notice crystals compactness. For additional details, please Scan QR Code in top
left corner of figure |
11. DISCUSSION
Prior research by this author showed catalase proper to be an emitter of electromagnetic radiation demonstrated Embi (2018) by using a novel tabletop optical microscopy methodology (TTM) able to detect incoming electromagnetic radiation Scherlag et al. (2016). The main component of the TTM is diluted Potassium Ferricyanide (K3Fe) crystals in drops of water. K3Fe exhibits “total absorption of incoming electromagnetic radiation” (EMFs) Baranov et al. (2015), Figgis et al. (1969), therefore, a fit to detect tissue energy in dead or alive biological samples via In Vitro experiments previously described in a paper published in 2016. In other words, when drops of K3Fe in solution detect incoming radiation, the steady progress of K3Fe crystallization and crystals densities is delayed and their compactness have been observed to be directly proportional to a shorter distance from the incoming electromagnetic radiation strength Embi (2020). This newly introduced observation is easily seen in Figure 6 above. The farther from the EMF source the weaker the signal, and crystals compactness. The repetition of organized K3Fe semi-circular crystals is seen as periodic emissions of EMFs by the tail tip. The same phenomenon (periodic emissions) was previously found emitted by freshly plucked hair follicles Figure 4.
12. SUMMARY AND CONCLUSIONS
Catalase has been identified as essential in tissue metabolism; its role in breaking down toxic chemicals during cell respiration is of paramount importance in cell survival. In this manuscript the presence of catalase is also demonstrated in dead tissue as evidence is presented on ≈ one-month dead lizard tissue fragment. Energy emitted by the dead tissue is displayed as precipitating crystals formation in liquid K3Fe on a glass slide. Images are presented documenting a direct relationship between EMFs intensity and K3Fe crystals distribution, in other words the weaker the EMFs the less dense precipitation of K3Fe crystals Figure 4, Figure 5, Figure 6, Figure 7, Figure 8 + videos). Biological tissue energy is shown to be present in dead and living similar tissue samples. Further research is advised.
13. Additional Commentary
As often happens in research unexpected additional information is documented; in this manuscript the distal tip of a reptile tail (lizard) is shown as a point of energy discharge of EMFs. This is appreciated when running the complete video recording in Figure 8. The distal end showing organized semi-circular concentric EMFs emissions, whereas the cut end lacks that characteristic. It could be stated “In reptiles, the tail tip is identified as a point where EMFs are emitted. Is the tail also a receiving point detecting external energy sources?
14. Questions Arise
First question:
What is the role of catalase in dead tissue?
In this manuscript a new finding is presented, the detection of energy stored in dead biological tissue attributed to catalase. Role unknown only hypothesized (see reply to question 2 below).
Second question:
If catalase has been identified as an essential in the living by neutralizing toxic reactive oxygen species; then what if any is its role in the dead?
In this manuscript, the presence of energy in dead tissue could be hypothesized to be a signalling mechanism attracting scavengers.
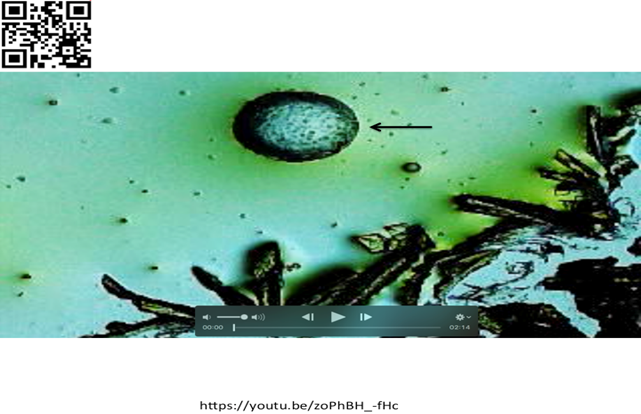
15. SUPPLEMENTARY BONUS INTERESTING CURIOUS FINDING SHOWING DISLODGED FAT CELL FROM PROXIMAL LIZARD TAIL EMITTING ENERGY DELAYING ADVANCE OF K3FE CRYSTALIZATION. ALSO, CRYSTALS PIERCING CELL OUTER MEMBRANE
Figure 9
|
Figure 9 Lizard tail shown in Exhibit I (Sample 3) immersed in drops of K3Fe. Round structure (Possible Fat Cell) migrating towards ADVANCING forming crystals. Crystals seen piercing outer membrane of structure- Also noticed is delay in crystallization advance, possibly indicating electromagnetic energy emitted by fat cell. For details link to: https://youtu.be/zoPhBH_-fHc Or Scan QR Code in left upper corner of
image. |
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
Baranov, D. G., Edgar, J. H. , Hoffman, T., Bassim, N., and Caldwell, J. D. (2015). Perfect Interferenceless Absorption at Infrared Frequencies by a Van Der Waals Crystal. Physical Review B, 92 (20). https://doi.org/10.1103/PhysRevB.92.201405
Embi, A. A. (2018). “Catalase Intrinsic Emissions Of Electromagnetic Fields As Probable Cause in Cancerogenesis from Consumption of Red and Processed Meat.” International Journal Of Research - Granthaalayah, 6(8), 33-40. https://doi.org/10.29121/granthaalayah.v6.i8.2018.1259
Embi, A. A. (2020). The Human Hair Follicle Pulsating Biomagnetic Field Reach as Possible Additional Factor in Migraine Headaches a Biophysics Based Hypothesis. International Journal of Research -Granthaalayah, 8(5), 221-229. https://doi.org/10.29121/granthaalayah.v8.i5.2020.179
Figgis, B. N., Gerloch, M., Mason, R., and Nyholm, R. S.(1969). The Crystallography And Paramagnetic Anisotropy of Potassium Ferricyanide. https://doi.org/10.1098/rspa.1969.0031
Scherlag, B. J., Sahoo, K., and Embi A. A. (2016). A Novel and Simplified Method for Imaging The Electromagnetic Energy in Plant and Animal Tissue. Journal of Nanoscience and Nanoengineering 2016, 2 (1), 6-9.
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2022. All Rights Reserved.