TEMPERATURE DEPENDENCE OF CONDUCTIVITY OF UREASE DOPED POLYPYRROLE MATERIAL
Abstract
In situ polymerization of pyrrole was carried out in the presence of urease to synthesize polypyrrole–urease composites by chemical oxidation method. The PPy/ urease have been synthesized with various compositions (10, 15, 20, 25 and 30 wt%) of urease in pyrrole in aquas medium at room temperature. The polypyrrole– urease composites were characterized by infrared spectroscopy (IR). The d.c. conductivity was studied in the temperature range from 40–100°C. The dimensions of urease in the matrix have a greater influence on the observed conductivity values.
Keywords
Polypyrrole, Urease, Composites, Conductivity
INTRODUCTION
Urea is observed in many natural products like urine, milk and blood serum, to find quality many times it is needed to be analyzed. Human health is directly connected to change in urea concentration of urea, in human body. The concentration of urea in human body ranges 1.7- 8.3 mmol/L which varies up to 100 mM/l under pathophysiological condition. Milk is biological sample which contains urea (Lima, Fernandes, & Rangel, 2004), and helps to understand animal health by counting amount of urea in milk, monitoring and prediction of diet protein needs. The understanding of D.C. conductivity of urea in pyrrole will help to get biosensor to monitor urea concentration in biological or biologically derived molecule such as liposomes antibodies, tissues, microorganisms, yeast, enzymes, antigens, , organelles and DNA etc. For Immobilization and electrode surface conduction conducting polymers are used (Kaneto & Kaneko, 2002; Sharma, Gerard, Singhal, Malhotra, & Annapoorni, 2001).
Conducting polymers have fascinated many scientist to use it as a suitable matrix of enzymes. Conducting polymers are used to improve bio polymers speediness, adaptability and sensitivity of biosensors in diagnostics to measure vital analytes. The conduction supporting polymers are good base material for entrapment or appropriate medium of enzymes. The flexibility of polypyrrole makes it suitable base matrix for augmenting chemical structure, electronic and mechanical properties.
MATERIAL AND METHOD
PREPARATION OF POLOYPYRROLE
PPY-Cl powder was acquired by in situ polymerization of pyrrole by using ferric chloride as oxidant. Dissolved in 300ml was stirred for 15 min, then 0.043 mol (3 ml) of pyrrole dissolved in 100 ml of distilled water was added to the stirred mixture. The oxidant/pyrrole molar ratio was 2:3 as recommended by Myers and Armes (Armes, 1987), which is the optimal value. The polymerization was carried out for one hour at room temperature with moderate stirring.
PREPARATION OF POLOYPYRROLE -UREASE
To above reaction mixture, varied weight percent of urease ( 10%,15%,20%,25% and 30 wt %) was added to form urease entrapped polypyrrole composites the polymerization was allowed to proceed for 6 hours at room temperature. Filtering and washing with distilled water obtained black precipitate. Obtained composites were dried at 60 for 24 hours. The Fourier Transform Infrared spectra was recorded using the NICOLET 6700 spectrometer in KBr medium. The D.C. conductivity was done by using home made two probe method.
RESULTS AND DISCUSSION
THE FTIR SPECTRUM OF UREASE ENTRAPPED POLYPYRROLE
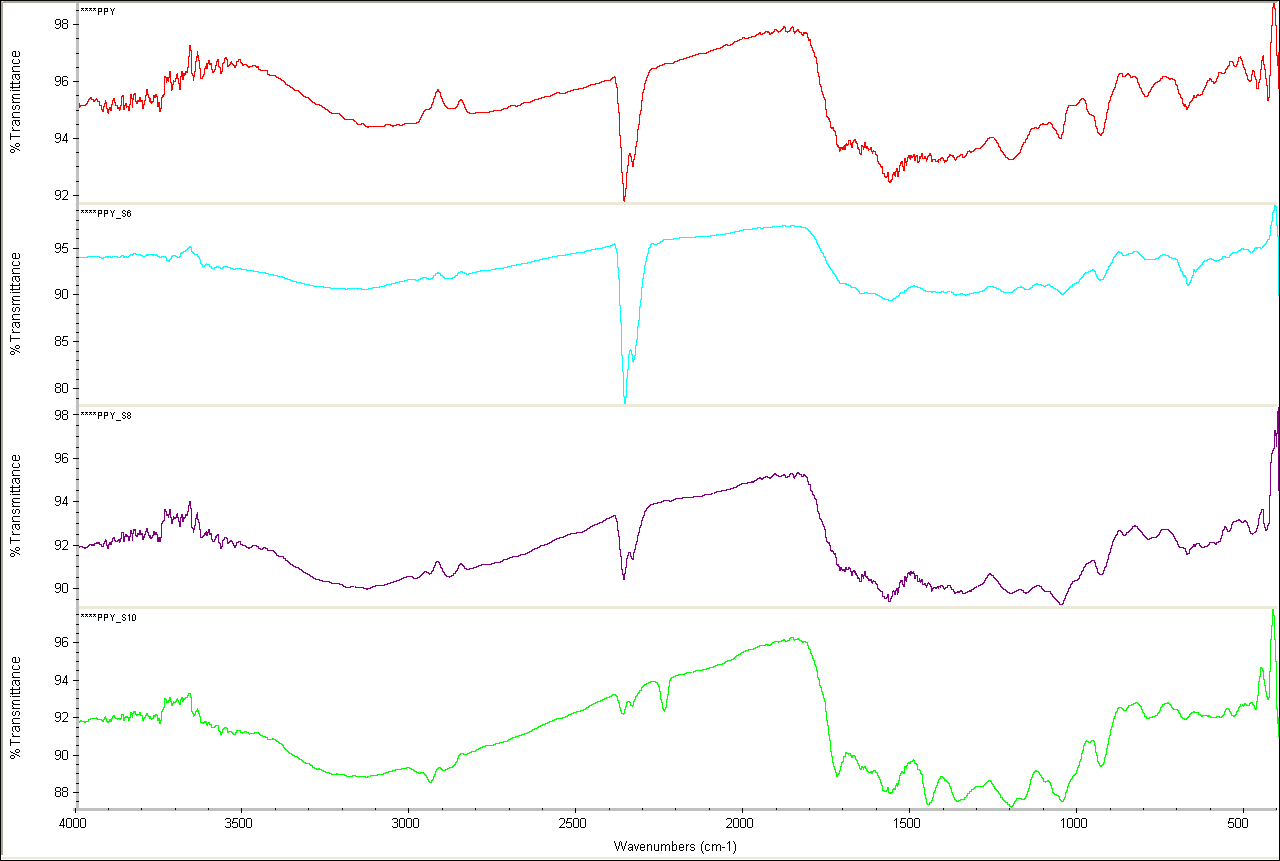
IN fig (Figure 1 ) the IR observation bands for polypyrrole were assigned according to the standard table. The existence of urease in the polypyrrole urease composite is established by the observation of urease bands in the spectra, 1655.5 cm‑1 in polypyrrole urease composite (20%) and 1725.6 cm-1 in polypyrrole urease composite (30%). The locations of peaks in the composites are marginally shifted in the spectra with reference to the spectrum of pure polypyrrole, which specifies a more intimate interaction within urease and polypyrrole.
The FTIR spectrum obtained for ppy shows the presence of characteristic absorption bands at 1567.4 cm-1, 1457.8 cm-1 ( C = C stretching of pyrrole ring) which is shifted to 1569 cm-1 in polypyrrole urease composite. The band of C-N stretching vibrations at 1403.7 cm-1 in the spectrum of pure ppy is shifted to 1339.5 cm-1 in polypyrrole urease composite (10%) and to 1345 cm-1 in polypyrrole urease composite (20%) and to 1355 cm-1 in polypyrrole urease composite (30%). 1203.9 cm-1 C-H in plane deformation, 1056.5 cm-1 N-H in plane deformation, 935.9 cm-1 C-H out of plane deformation and 677.4 cm-1 C-C out of plane ring deformation or C-H rocking.
CONDUCTIVITY OF UREASE DOPED POLYPYRROLE
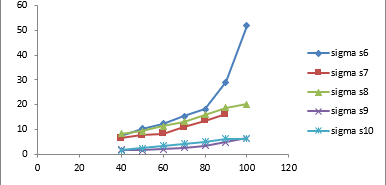
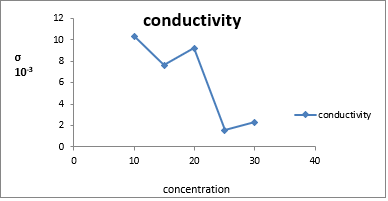
The conductivity graph of σ d.c.scm-1 10-3with temperature for ppy composite is shown in the fig (Figure 2 ).The conductivity decreases as concentration of urease increases in the samples with urease percentage of the composites ( 10,15, 20,25 and 30 wt. % ).It shows urease particles are entrapped inside ppy chain. for higher (25%,30%) ratio of urease the connectivity of polypyrrole due to entrapped particle conductivity reduces. fig (Figure 3 ) shows σ d.c.scm-1 at 500c and concentration for composites ( 10,15,20,25 and 30 wt. % )maximum conductivity is observed at lower concentration .
CONCLUSION
FTIR spectroscopy reveals the presence of urease in polypyrrole urease composites by the observation peaks of urease. The positions of peaks in the composites are slightly shifted in the spectra with comparison to the spectrum of pure polypyrrole, which indicates a more intimate interaction between the urease bands in the spectra. The Electrical characterization of Polypyrrole and its urease composites (with varied wt. % of urease 10%, 20%, 30%) ,prepared by chemical oxidation method in the room temperature is in this study. The electrical characterization (d.c.) shows that as doping increases, the conductivity decreases ,due to unsymmetrical distribution and the increased amount of urease causes loss of conductivity. The conductivity v/s temperature graph shows reduction in slope as concentration of urea increases may be due to change in conjugation length.
It is also possible that thermal curling affects PPy chain alignment, which could cause increasing conjugation length and decreases resistivity of the sample

