STRESS TOLERANCE AND ANTIBIOTIC SUSCEPTIBILITY OF CRONOBACTER SPP. ISOLATED FROM POWDERED INFANT FORMULA RETAILED IN NIGERIA
Abstract
Cronobacter spp., is an emerging, opportunistic pathogen that causes infections such as septicaemia, meningitis and necrotizing enterocolitis in neonates and infants, and can sometimes lead to death. There is zero tolerance for the presence of Cronobacter spp. in all powdered infant formulae because of the high mortality rate (80%) associated with Cronobacter spp. infections. Three Cronobacter spp. (CS14, CS17 and CS124) isolated from PIF retailed in Nigeria were exposed to different levels of stress (pH, osmotic, oxidative, heat, bile and desiccation). The biofilm production ability of the isolates was investigated and the susceptibility of the isolates to different antibiotics was carried out using the Microscan MIC panel. Variation in stress response was observed in the isolates with no consistent pattern. The CS 17 (Cronobacter sakazakii) and CS 124 (Cronobacter sakazakii) showed the highest tolerance to stress on the average. All the isolates exhibited the ability to produce biofilm ranging from 1.30 – 2.0 absorbances and were also sensitive to more than 95% of the antibiotics used in the Microscan MIC panel, with no resistance to any.
Keywords
Cronobacter, Powdered Infant Formula, Stress, Antibiotic Susceptibility
INTRODUCTION
Cronobacter spp. is an emerging opportunistic food-borne pathogen that has been associated with serious infections in neonates and infants as well as elderly and immuno-compromised adults (Tall et al., 2015). Infantile disease presents clinically as meningitis, septicaemia or bacteraemia, and necrotizing enterocolitis in new-borns, particularly affecting premature or other immunocompromised infants (Alsonosi et al., 2015), (Farmer, 2015), (Holý & Forsythe, 2014), (Hunter & Bean, 2013).
Studies have shown that Cronobacter spp contaminate a lot of foods including infant foods (powdered infant formula, follow-up formula), dried milk protein products, cheese, liquorice, candies, dried spices, teas, nuts, herbs, vegetables, filth and stable flies, and powdered infant formula (PIF) or in milk powder production facilities and household environments (Berthold-Pluta, Garbowska, Stefańska, & Pluta, 2017), (Yan et al., 2015).
Powdered infant formula (PIF) milk has been epidemiologically linked to disease outbreaks caused by Cronobacter sakazakii (Brengi et al., 2012), (Farmer, 2015), (Pagotto & Farber, 2009). The addition of non-heat-treated materials and environmental contamination during filling and packaging are the plausible causes of Cronobacter contamination of PIF since Cronobacter strains cannot survive after the standard pasteurization procedures (Nazarowec-White & Farber, 1997). Foodborne pathogens must overcome several hurdles after they enter the host to be able to establish and cause infection. However, the body defense mechanisms prevent serious infections. These include the strong acid condition in the stomach, the presence of bile salts, antimicrobial peptides, and other hostile conditions. Cronobacter sakazakii exhibits unusual resistance to growth conditions such as acid stress and growth at minimum pH values of ∼4.5, although this value varies depending on the strain and type of acid (Alvarez-Ordonez, Begley, & Hill, 2012). Bile is an important antimicrobial component of the human digestive system, but growth has been observed for some C. sakazakii isolates at bile salt concentrations as high as 5% (Fakruddin et al., 2013). Cronobacter sakazakii is reported to be significantly more thermotolerant when compared with other members of the family Enterobacteriaceae (Amalaradjou, Kim, & Venkitanarayanan, 2014). This feature gives it a competitive advantage thereby facilitating its survival during improper PIF reconstitution (Bai, Yu, Guo, Fei, & Shi, 2019).
Cronobacter spp. appears to differ considerably in terms of their susceptibility to various antibiotics compared to many enteric bacteria. Antibiotic therapy is considered to be the common and preferred way to prevent the Cronobacter infection in humans (Depardieu, Podglajen, Leclercq, Collatz, & Courvalin, 2007). Many studies have confirmed that Cronobacter strains can be effectively eliminated by antibiotics (Hoque et al., 2010), (Fei et al., ) however, prolonged use of antibiotics is undesirable as it may result in the development of Cronobacter antibiotic resistance strains (YONEYAMA & KATSUMATA, 2006), (McMahon, Xu, Moore, Blair, & McDowell, 2007).
This study aimed at evaluating the survival strategies of Cronobacter spp. exposed to different stress conditions posed by pH, temperature, osmotic potential, oxidative, heat, high bile concentrations, desiccation as well as antibiotics susceptibility.
MATERIALS AND METHODS
Stress Tolerance of Cronobacter spp.
Three Cronobacter spp. were isolated from a total of 154 powdered infant formula retailed in Nigeria (Ezeh, Aboaba, Murray, Tall, & Smith, 2016). All stress tolerance experiments on the isolates were performed in Luria-Bertani (LB) broth (Difco). Stationary-phase cell suspensions were obtained by inoculating 10 ml of fresh Luria-Bertani (LB) broth with an isolated colony of each bacterium and incubated overnight at 37oC. When required, the challenge medium was supplemented by adding 3 N HCl (Sigma), 3 N NaOH (Merck), or 30% H2O2. Heat treatments were carried out at 60°C. Once the temperature of the treatment medium (1 ml of LB) was stabilized, an inoculum of 0.01 ml of each bacterial suspension was added. During heating, samples were removed and plated on Luria-Bertani (LB) agar. For acid, alkaline, and oxidative treatments, aliquots of stationary-phase cultures were inoculated (1% v/v inoculation concentration) into Luria-Bertani (LB) broth supplemented with HCl (pH 2.5), NaOH (pH 11.0), or H2O2 (30 mM). After incubation at room temperature, the survival rate was monitored periodically.
The effect of desiccation on the cells, was assessed by transfering 100-μl aliquots of stationary-phase cultures into 96-well culture plates (Falcon). They were kept without lids in a 25°C incubator for air drying. Under these conditions the sample was kept for approximately 3 h. Subsequently, plates were incubated at room temperature for up to 8 days, and bacterial survival pattern was determined after the rehydration of samples with addition of 100 μl PBS.
The effect of osmotic stress was determined by inoculating, aliquots of stationary-phase cultures (1% v/v inoculation concentration) into Luria-Bertani (LB) broth supplemented with NaCl (8%).
Growth was determined on all occasions by preparing 10-fold serial dilutions in sterile Phosphate Buffered Saline (PBS) solution and suitable dilutions plated in duplicates on Luria-Bertani (LB) agar plates.The plates were incubated at 37°C for 24 - 48 h and viable cells counted and survival rate (%) determined.
Bile salts resistance assay
To determine the bacterial resistance to bile salts, bacterial isolates were grown in Luria-Bertani (LB) broth for 24 h at 37oC. From these 20 µl was then inoculated into 1 ml (1: 50) of fresh Luria-Bertani (LB) broth containing 0.3% bile salts. After 60 min and 24 h incubation at 37oC, bacterial isolates were serially diluted and plated out on LB agar. The percentage survival of each isolate was calculated.
Biofilm Formation of Cronobacter Isolates
The isolates were sub cultured overnight on Brain Heart Infusion (BHI) agar. Each was grown in Tryptic Soy broth (TSB) without glucose at 37oC with agitation starting 16 to 24 h before inoculating into 96-well micro titre plates. Dilution (1: 100) of the overnight grown cultures in Tryptic Soy broth (TSB) was carried out and 200 μl of each bacterial suspension was inoculated into sterile 96-well polystyrene micro titre plates (Ref BD# 353072). Micro titre plates were incubated for 24 h at 37oC. After 24 h incubation, plates were washed gently three times with 200 μl of phosphate buffered saline (PBS pH 7.3) using a multichannel pipette and the wells were emptied by flickering the plates. Bacteria were fixed with 200 μl of Bouin’s fixative for 30 min at room temperature in the dark, and then rinsed once with phosphate buffered saline (PBS). The fixed bacterial cells were stained with 200 μl of 1% crystal violet for 30 min at room temperature in the dark, and then rinsed thoroughly with distilled water (4-5 times). The plates were air dried for 15 min and the cells were solubilized in 200 μl of ethanol: acetone (80:20, v/v). After 15- 20 min, the optical density at 570 nm was measured using a micro plate reader (Mullikan Spectrum, Thermo LabSystem).
Antibiotic Susceptibility pattern of Cronobacter Isolates
Using the same micro-swab and membrane used for identification by MALDI-TOF VITEK MS RUO, a suspension was created following the Lysis-Filtration process and set aside until an identification result was obtained from the VITEK MS. As cellular debris is lysed and filtered, bacteria left on the filter is used for suspension preparation. The suspension was then adjusted to a McFarland standard of 0.5 and used for direct antibiotic susceptibility testing on VITEK®2 System. VITEK®2 cards were inoculated following manufacturers instruction. The ID from VITEK MS was introduced into the VITEK®2 system to allow it to choose the correct interpretive criteria. The resulting MIC was translated into clinical categories of susceptible, intermediate, or resistant following the Clinical and Laboratory Standards Institute (CLSI) recommendations (M45-A2/ M100-S22). The antibiotics used were amikacin (<16 µg/ml), amp/sulbactam (<8/4 µg/ml), ampicillin (<8 µg/ml), aztreonam (<4 µg/ml), cefepime (<4 µg/ml), cefotaxime (<2 µg/ml), ceftazidime (<1 µg/ml), ceftriaxone (<8 µg/ml), cefuroxime(<4 µg/ml), cefoxitin (<8 µg/ml) ciprofloxacin (<1 µg/ml) doripenem (<0.5 µg/ml), ertapenem (<1 µg/ml), gentamicin (<4 µg/ml), levofloxacin (<2 µg/ml), meropenem (<1 µg/ml), piperacillin-tazobactam (<16 µg/ml), tetracycline (<4 µg/ml), tigecycline (<1 µg/ml), tobramycin (<4 µg/ml), trimethoprim/sulfamethoxazole (<2/38 µg/ml) and cefazolin (16 µg/ml).
RESULTS
Stress Response of Cronobacter Isolates
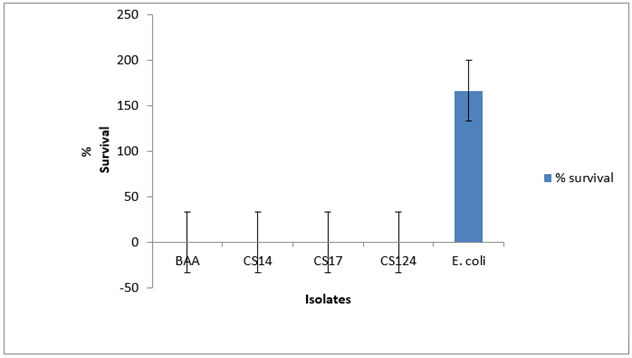
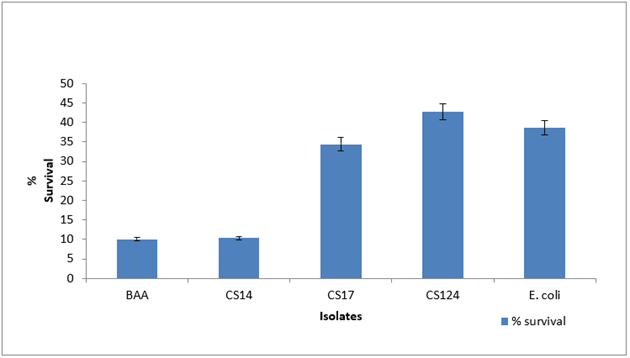
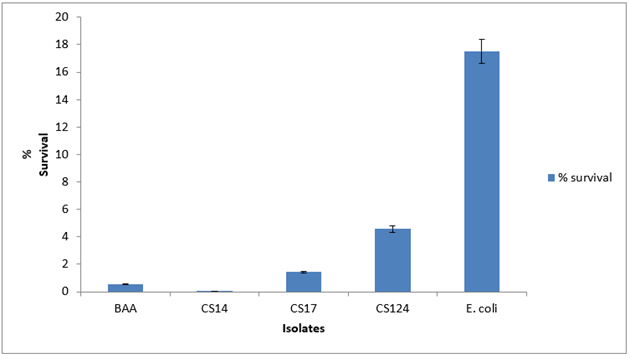
Variation in stress tolerance was observed with no consistent pattern. For acid tolerance among the Cronobacter spp., the survival rate was less than 1% for 60 min as shown in Figure 1 . At pH 11, CS 17 showed a survival rate of 34% while CS 124 survived at 43% as shown in Figure 2 . CS 14 had a survival of just 10%. At 30mM H2O2 concentration for 60 min, only CS 124 survived with 0.2%. This is shown in Figure 3 . At 60oC for 5 min, survival ranged from <1-4.6%. CS 14 was the most sensitive at this temperature while CS 124 was the most resistant. This is shown in Figure 4 . All the isolates showed good tolerance to desiccation after eight days as shown in Figure 5 , with CS 124 having the highest survival percentage of about 75%.
At 8% NaCl concentration for 60 min, all isolates survived well with a range from 36-55% as shown in Figure 6 . Bile concentration at 0.3% for 24 h had no negative effect on all isolates as shown in Figure 7. The isolates were able to grow under this concentration.




Biofilm Formation of Cronobacter Isolates
All the isolates demonstrated very good abilities to produce biofilm ranging from about 1.3 – 1.7 at OD 570nm as shown in Figure 8.

Antibiotic susceptibility Testing
All the isolates were sensitive to most of the antibiotics (>95%) tested based on the Interpretive Breakpoints as indicated in Clinical and Laboratory Standards Institute (CLSI) document M45-A2 or M100-S22. Intermediate response to Cefazolin at an MIC of 16 µg/ml was observed as shown on Table 1 . There was no resistance observed.
|
Drug |
MIC (µg/ml) |
Interpretation |
|
Amikacin |
< 16 |
S |
|
Amp/Sulbactam |
< 8/4 |
S |
|
Ampicillin |
< 8 |
S |
|
Aztreonam |
< 4 |
S |
|
Cefepime |
< 4 |
S |
|
Cefotaxime |
< 2 |
S |
|
Ceftazidime |
< 1 |
S |
|
Ceftriaxone |
< 8 |
S |
|
Cefuroxime |
< 4 |
S |
|
Cefoxitin |
< 8 |
S |
|
Cefazolin |
16 |
I |
|
Ciprofloxacin |
< 1 |
S |
|
Doripenem |
< 0.5 |
S |
|
Ertapenem |
< 1 |
S |
|
Gentamicin |
< 4 |
S |
|
Levofloxacin |
< 2 |
S |
|
Meropenem |
< 1 |
S |
|
Pip/Tazo |
< 16 |
S |
|
Tetracycline |
< 4 |
S |
|
Tigecycline |
< 1 |
S |
|
Tobramycin |
< 4 |
S |
|
Trimeth/Sulfa |
< 2/38 |
S |
DISCUSSION
The gastric microbial barriers of the stomach and small intestine decrease the chance of colonization by pathogens but do not provide protection against bacteria that have adapted to survive within these extremely harsh conditions. Enteric bacteria have mechanisms that allow for survival and proliferation within the human gut (Merritt & Donaldson, 2009). Cronobacter exhibit unusual resistance to heat, desiccation and acid stress growth conditions compared with other members of the family Enterobacteriaceae (Dancer, Mah, Rhee, Hwang, & Kang, 2009). The ability of Cronobacter spp. to withstand these stresses is crucial for their survival, persistence and infectivity. CS 14, CS 17 and CS 124 have demonstrated tolerance to several food- and host-related stresses (acid, alkaline, osmotic, oxidative, and heat stresses, and desiccation).
The gastric pH of infants six days old varies with age from a fasting pH of 2.9 to a value of 5.2 directly after eating (Holton et al., 2014). As the infants gets older between 7-15 days old, the gastric pH ranges between 4.6 and 5.8. CS 14 (C. malonaticus) was the most resistant at pH 2.5 for 60 min with a survival rate of less than 1%. Wan-Ling et al. (2010) reported decline in the populations of C. sakazakii when exposed to gastric juice with pH 2.0 - 4.0 and an increase in pH led to an increase in the survival percentage. Because the pH of the gastric juice does not always remain low, there is a high possibility of Cronobacter surviving and causing an infection. At pH 11, all the isolates survived with CS 124 (C. sakazakii) having the highest survival of 43%.
Cronobacter sakazakii is well known to be resistant to osmotic and dry stress (Feeney & Sleator, 2011). All isolates in this study survived 8% NaCl for 60 min with a range of 36-55%. The C. sakazakii isolates had a higher capacity to grow in the hyperosmotic media, in agreement with previous studies (Fei et al., ), (Alvarez-Ordonez et al., 2012). All the isolates showed resistance to 8 days of desiccation. This high tolerance to osmotic stress and desiccation may provide a competitive advantage in dry environments, such as is found in powdered infant formula.
Bile is an important antimicrobial component of the human digestive system and Gram-negative bacteria are inherently resistant to it. There was an increase in the viable population of all the isolates in this study at 0.3% bile concentration. This supports the work of (Hsiao, Ho, & Chou, 2010), which reported an increase in the viable population of Cronobacter sakazakii after exposure to 0.5% and 2.0% bile salt solution for 12 h. The ability of enteric bacteria to survive in the presence of large quantities of bile salts is directly related to their ability to establish invasive infections (Crawford, Gibson, Kay, & Gunn, 2008).
Temperature abuse of reconstituted formula is a common risk factor in reported Cronobacter sakazakii outbreaks (Caubilla-Barron et al., 2007); hence the need for temperature control to reduce microbial growth in reconstituted formula. At a temperature of 60oC for 5 min, CS 124 (C. sakazakii) was the most resistant while CS 14 (C. malonaticus) was the most sensitive. (Arroyo, Condón, & Pagán, 2009) reported that the heat resistance of Cronobacter spp. varies widely among strains. (Huertas et al., 2015) reported that when artificially inoculated powdered infant formula (PIF) was reconstituted at different water temperatures (50, 55, 60, 65, 70°C) and cooled at different rates, C. sakazakii survived for long time periods in powdered formula and proliferated after reconstitution. Water at temperatures between 50 and 65°C for reconstitution did not provide a significant inactivation of C. sakazakii cells. This supports the result of this present study. The rise in Cronobacter notoriety has prompted changes in the microbiological criteria for powdered infant formula (PIF) and reconstitution procedures. The FAO/WHO document (WHO, 2007) “Guidelines for the safe preparation, storage and handling of powdered infant formula” recommends reconstituting with water which has cooled to 70°C from boiling in order to destroy vegetative cells, reconstitute only the required amount and reduce the storage period prior to consumption as much as possible (, 2007).
Cronobacter have been reported to attach to and from biofilms on stainless steel, glass, latex, silicon, polyvinyl chloride and polycarbonate (Iversen, Lane, & Forsythe, 2004). The biofilm serves as physical protective barrier from environmental stresses as well as host immune resistance mechanisms (, 2006). In this study, all the isolates demonstrated very strong abilities to produce biofilm ranging from 1.3 - 1.7 absorbance at OD 570 nm. Biofilm formation is of special importance in the food industry because biofilms can act as a source of microbial contamination that might lead to spoilage of foods or contamination of food products undergoing processing (Hartmann et al., 2010).
The antibiotic susceptibility pattern of the strains revealed that all the isolates were susceptible to amikacin, amp/sulbactam, ampicillin, aztreonam, cefepime, cefotaxime, ceftazidime, ceftriaxone, cefuroxime, doripenem, ertapenem, gentamicin, levofloxacin, meropenem, piperacillin-tazobactam, tetracycline, tigecycline, tobramycin, and trimethoprim/sulfamethoxazole with MIC ranging from < 0.5 - < 16 µg/ml. Being susceptible to majority of the antibiotics used (> 95%) in this study will make the treatment of infections caused by any of the isolates effective. Intermediate response to cefazolin at 16 µg/ml was also observed in this study. This result is supported by (Fei et al., ) who reported the antimicrobial susceptibility of 70 Cronobacter strains, representing 19 sequence types. Although multiple antibiotic resistance (mar) operons were found in Cronobacter (Burgos & Varela, 2002), the overall level of antibiotic resistance was low when compared with other food-borne pathogens.
CONCLUSIONS AND RECOMMENDATION
The use of hygienic measures during preparation and reconstitution of powdered infant formula (PIF) are essential. In addition, infant formula producers must enforce the use of guidelines aimed at decreasing the risks of product contamination with foodborne pathogens. The control of primary populations of Cronobacter spp. during the powdered infant formula (PIF) production process and prevention of post processing contamination can be ensured by using suitable microbiological guidelines for quality control and assurance. Sanitary practices for the preparation of infant formula in both the home and hospitals should be carefully controlled through the regular creation of the awareness that powdered infant formula (PIF) are not sterile but that they may contain potential pathogens.

