OUTCOME BASED LEARNING: A PANACEA FOR QUALITY EDUCATION
Abstract
The curriculum framework asserts learner to debate, dissent form individual opinion on ideas, systems, practices by nurturing skills to think and reason independently. Participatory learning activity is envisioned as a process whereby learners construct concept through assimilation, absorption, interaction and reflection. In the present paper experiential learning activity are designed to ensure that the students are encouraged to seek out knowledge from their hands-on activity than the text book in their own experiences. In the present paper attempt is made to investigate the impact of conventional method of teaching and experiential learning activities on attainment of knowledge and retention of knowledge in learning redox reaction. Data is collected by using pre-test, post-test and retention test. Instrument is validated by experts. Stratified random method is applied to draw the sample. Totally 50 students participate in the study. Sample is separated into two: control and the study group. Control group is exposed to the conventional chalk and talk method while the students from the study group are exposed to Experiential Learning Activities. The result of the study reveals that there is noteworthy difference in the mean score in learning redox reaction in the scores of pre-test, post-test and retention test between the study group and the control group.
Keywords
Redox Reaction, Interest, Experiment, Experiential Learning and Hands-On Activity
INTRODUCTION
“All birds find shelter during rain, but eagle avoid rain by flying above the clouds. Problems are common but attitude make the difference” – Dr. A.P.J. Abdul Kalam
Experiential learning – learning through exploration, discovery, experience, doing and acting. Learning content is important but learning through process is the heart of experiential learning. Experiential learning is learning through reflection on doing. One of the important activities for experiential learning is Hands-on activity, but basically, it does not include students on the products. It is a well-known and tested model for teaching-learning, training skills, facilitation, coaching and organizational development. It focuses on active participation of the learners in teaching learning process. It also focuses on the real-world application of skills and knowledge to the real world experience to further increase learners knowledge and nurture competence, skills and behaviour.
The notion of experience based learning is explored in teaching learning by John Dewey, Kurt Han , Kurt Lewin, jean Piaget amongst others but it is made popular by David A Klove.
Definition
• It may be subject- focused and work focused.
• Stimulation, collaboration, exchange of ideas and prospective.
• Essential active participation in making construction.
• It’s foundation is based on past experience and knowledge.
• It is amalgamation of experience and focus reflection.
The foundation of the model for experiential learning cycle is “Do-Reflect and Decide”, David Klove in 1994 define experiential learning as the process whereby knowledge is created through the transformation of experience. The most noteworthy and pervasive goal of the school education is to teach ‘learn to think’. In this context, Science subject contributes its unique skills with its emphasis on manipulation, hypothezing physical world and reasoning from data.
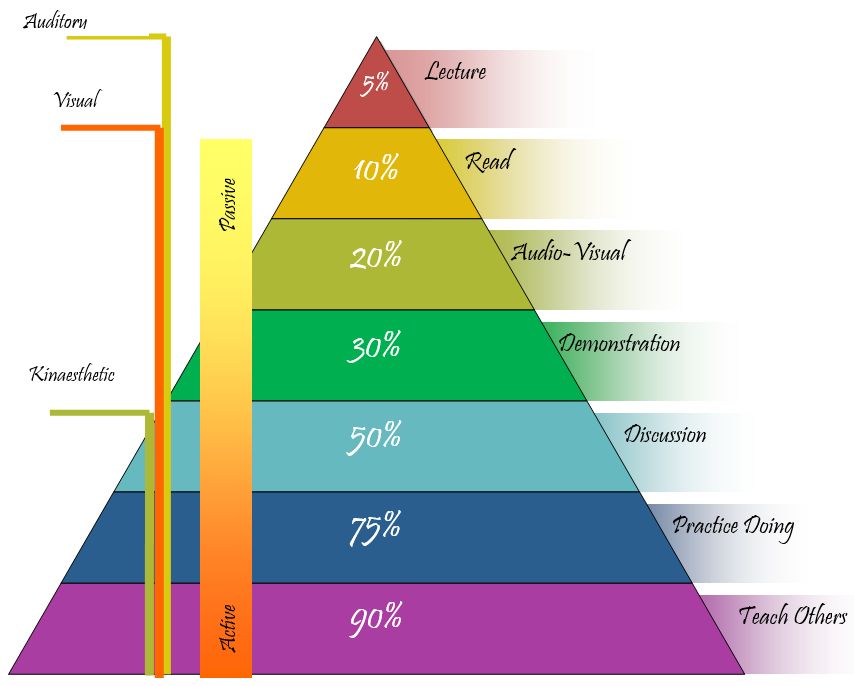
CONE OF LEARNING
Study shows that material and methods enhance students recall of information, retention and managing learning experience. According to the cone of learning proposed by National training laboratories suggest that learners only remember 10% from reading books and 90% when they learn through experiential learning activities.
Lecture is the most ineffective and traditional, conventional model of teaching and memorization of information. It is a passive form of learning and students are the passive listeners for information being spoon fed by the teacher. Audio learners encourage lecturer method. It is effective when learners arrive to the class well prepared, actively participate in note taking and class room discussion. Reading has more impact than the lecture method but it is less effective for acquiring and retention of information. Students first learn to read and then read to learn. Visual learners reading method is more effective as compared to non-visible learners. In our educational system reading textbook is mandatory and essential.
Audio-visual method only helps to retain 20% of retention of information. It includes various tools such as sound, video, pictures and graphs. It is effective when it aligned with other methods of teaching.
TYPES OF AUDIO-VISUAL METHOD
-
Demonstration- It provides learning task to the students and active participation in learning. It provides ambiguity than passive study method and minimizes the chances of misconception and fosters deep conceptual understanding.
-
Discussion- It is a co-operative and active form of learning with better and greater retention of information and catalyzes higher academic achievement. It fosters thinking, participation and engagement in teaching-learning process.
-
Discovered Learning- It is the most effective method to enhance sound conceptual understanding and retention of information for the longer time.
-
Peer Teaching- The mastery over the content is the key for accurate and correct teaching. It is effective to attain higher recall and retention power. It retains 90% of information.
CONCEPTUAL FRAMEWORK
The “Redox Reaction” is considered as the most difficult unit not only for learning but also for teaching. Lack of conceptual understanding will hinder the students conceptual understanding. Traditional chalk and talk does not foster deep understanding. The concept of Redox reaction is difficult for learners due to its abstract nature and few anomaly’s in microscopic words. The term ‘equilibrium’ is closely related to the everyday concept of equality and balance and create confusion amongst the learners. Students lack conceptual understanding of its underlined principles. The concepts are complex and integrated with constructivism. Teaching has to be done by following certain principles in order for the learners to achieve intended specific learning outcome.
Redox indicator catalyzes the reducing agent of auto-oxidation. The most popular experiment is experiment of blue bottle. In alkaline medium oxidation of glucose is oxidized by Methane blue indicator. During chemical reaction methylene blue is reduced to colourless compound (leucon) by aldose sugar when on shanking and after five minutes when the solution settles down it oxidizes to blue colour by the presence of atmospheric oxygen. This cycle can be frequently done before the reaction runs out of time or before the solution turns brown due to side reaction.
This experiment may be repeated with ascorbic acid and benzoic acid as reducing agent with various dyes such as safrene, resazurin, erioglauncine, tetrazolium Chloride. The aldehydes along with the colour bottle reaction is the most common demonstration- due to its visual appeal, simplicity and readily available materials for reaction. The oxidation of reducing agents is grouped as:
-
Primary alcohol aldehyde
-
Secondary alcohol Ketones
-
Aldehyde carboxylic acid
MATERIALS REQUIRED
-
Distilled water as a medium for reaction.
-
Alkaline medium KOH/NaOH
-
Glucose/Ascorbic acid/ benzoic acid as reducing agent
-
Indicators – Methylene blue, safrene, resazurin, erioglauncine, tetrazolium Chloride.
-
One liter glass container (conical flask / flat bottom flask)
-
Spatula
O2(gas) O2 (dissolved)
O2 (dissolved) + methylene blue (clolurless) Methylene blue (blue)
Glucose + OH- Glucoside.
Glucoside + methylene blue (blue) methylne blue (colourless) + OH-
In this reaction glucose (aldehyde) in an alkaline solution is slowly oxidized by dioxygen to form gluconic acid.
CH2OH – CHOH-CH-OH-CHOH-CHOH-CHO+HOH CH2-OH -CH-OH-CH-OH-CH-OH-COOH
Gluconic acid is changed into Sodium gluconate in existence of Sodium hydroxide. Methylene blue is spread in the reaction by active oxygen as a transferring agent. On oxidizing glucose, methylene blue is reduced by forming leucon methylene blue and become colourless.
Sufficient oxygen is present in the air and leucon methylene blue is re-oxidized and blue colour of the solution is returned. On standing glucose is reduced to methylene blue dye and the blue colour of the solution disappear. In dilute solutions, reaction happens at around 40-600C and more concentrated solution at room temperature.
|
Dye 1: Methylene Blue |
Dye 2: Indio Carmine (Colour Change reaction) |
|
• Take 15 gm of glucose as reducing agent in 750ml of distilled water (Solution A). • Take 250 ml of distilled water and add 7.5gm of Sodium hydroxide (Solution B) • Warm the solution up to 600 C • Add pinch of Methylene Blue to the Solution A. Use small quantity of dye enough to make solution blue. • Add solution B to A. Addition of solution results in change of colour from blue to colourless solution • Pour this solution from the height of 60 cm in to empty beaker. It is vital to dissolve oxygen from air to the solution. The colour of the solution turn blue again. • This experiment may be repeated numerous times. |
• Take 15 gm of glucose as reducing agent in 750ml of distilled water (Solution A). • Take 250 ml of distilled water and add 7.5gm of Sodium hydroxide (Solution B) • Warm the solution up to 600 C • Add pinch of Indio carmine (disodium salt of Indio 5-5- dysphonic acid) to the Solution A. Use small quantity of dye sufficient to make solution blue. • Add solution B to A. Addition of solution results in change of colour from blue to green and over time the green colour change to red/golden yellow. • Pour this solution from the height of 60 cm in to empty beaker. It is vital to dissolve oxygen from air into the solution. The colour of the solution turn green. • Once again, the colour of the solution turn into red/golden yellow.. This experiment may be repeated numerous times. |
|
Dye 3: Safrene |
Dye 4: resazurin |
|
• Take 15 gm of glucose as reducing agent in 750ml of distilled water (Solution A). • Take 250 ml of distilled water and add 7.5gm of Sodium hydroxide (Solution B) • Warm the solution up to 600 C • Add pinch of safrene to the Solution A. Use small quantity of dye sufficient to make solution pink. • Add solution B to A. Addition of solution results in change of colour from dark pink to light pink. • Pour this solution from the height of 60 cm in to empty beaker. Vigorous pouring from the height is essential to dissolve oxygen from air into the solution. The colour of the solution turn from light pink to dark pink. • This experiment may be repeated numerous times. |
• Take 15 gm of glucose as reducing agent in 750ml of distilled water (Solution A). • Take 250 ml of distilled water and add 7.5gm of Sodium hydroxide (Solution B) • Warm the solution up to 600 C • Add pinch of resazurin to the Solution A. Use small quantity of dye sufficient to make solution pink. • Add solution B to A. Addition of solution results in change of colour from dark pink to light pink. • Pour this solution from the height of 60 cm in to empty beaker. Vigorous pouring from the height is essential to dissolve oxygen from air into the solution. The colour of the solution turn from light pink to dark pink. • This experiment may be repeated numerous times. |
|
Dye 5: erioglaucine |
Dye 6: Tetrazolium Chloride |
|
• Take 15 gm of glucose as reducing agent in 750ml of distilled water (Solution A). • Take 250 ml of distilled water and add 7.5gm of Sodium hydroxide (Solution B) • Warm the solution up to 600 C • Add pinch of erioglaucine to the Solution A. Use small quantity of dye sufficient to make solution blue. • Add solution B to A. Addition of solution results in change of colour from dark blue to light blue. • Pour this solution from the height of 60 cm in to empty beaker. Vigorous pouring from the height is essential to dissolve oxygen from air into the solution. The colour of the solution turn from light blue to dark blue.. • This experiment may be repeated numerous times. |
• Take 15 gm of glucose as reducing agent in 750ml of distilled water (Solution A). • Take 250 ml of distilled water and add 7.5gm of Sodium hydroxide (Solution B) • Warm the solution up to 600 C • Add pinch of tetrazolium chloride to the Solution A. Use small quantity of dye sufficient to make solution red. • Add solution B to A. Addition of solution results in change of colour from dark red to light red. • Pour this solution from the height of 60 cm in to empty beaker. Vigorous pouring from the height is essential to dissolve oxygen from air into the solution. The colour of the solution turn from light red to dark red.. • This experiment may be repeated numerous times. |
|
Dye 1: Methylene Blue |
Dye 2: Indio Carmine (Colour Change reaction) |
|
• Take 15 gm of benzoic as reducing agent in 750ml of distilled water (Solution A). • Take 250 ml of distilled water and add 7.5gm of Sodium hydroxide (Solution B) • Warm the solution up to 600 C • Add pinch of Methylene Blue to the Solution A. Use small quantity of dye sufficient to make solution blue. • Add solution B to A. Addition of solution results in change of colour from blue to colourless solution • Pour this solution from the height of 60 cm in to empty beaker. Vigorous pouring from the height is essential to dissolve oxygen from air into the solution. The colour of the solution turns blue again. • This experiment may be repeated numerous times. |
• Take 15 gm of benzoic as reducing agent in 750ml of distilled water (Solution A). • Take 250 ml of distilled water and add 7.5gm of Sodium hydroxide (Solution B) • Warm the solution up to 600 C • Add pinch of Indio carmine (disodium salt of Indio 5-5- dysphonic acid) to the Solution A. Use small quantity of dye sufficient to make solution blue. • Add solution B to A. Addition of solution results in change of colour from blue to green and over time the green colour change to red/golden yellow. • Pour this solution from the height of 60 cm in to empty beaker. Vigorous pouring from the height is essential to dissolve oxygen from air into the solution. The colour of the solution turns green. • Once again, the colour of the solution turns into red/golden yellow. This experiment may be repeated numerous times. |
|
Dye 3: Safrene |
Dye 4: resazurin |
|
• Take 15 gm of benzoic acid as reducing agent in 750ml of distilled water (Solution A). • Take 250 ml of distilled water and add 7.5gm of Sodium hydroxide (Solution B) • Warm the solution up to 600 C • Add pinch of safrene to the Solution A. Use small quantity of dye sufficient to make solution pink. • Add solution B to A. Addition of solution results in change of colour from dark pink to light pink. • Pour this solution from the height of 60 cm in to empty beaker. Vigorous pouring from the height is essential to dissolve oxygen from air into the solution. The colour of the solution turns from light pink to dark pink. • This experiment may be repeated numerous times. |
• Take 15 gm of benzoic acid as reducing agent in 750ml of distilled water (Solution A). • Take 250 ml of distilled water and add 7.5gm of Sodium hydroxide (Solution B) • Warm the solution up to 600 C • Add pinch of resazurin to the Solution A. Use small quantity of dye sufficient to make solution pink. • Add solution B to A. Addition of solution results in change of colour from dark pink to light pink. • Pour this solution from the height of 60 cm in to empty beaker. Vigorous pouring from the height is essential to dissolve oxygen from air into the solution. The colour of the solution turns from light pink to dark pink. • This experiment may be repeated numerous times. |
|
Dye 5: erioglaucine |
Dye 6: Tetrazolium Chloride |
|
• Take 15 gm of benzoic acid as reducing agent in 750ml of distilled water (Solution A). • Take 250 ml of distilled water and add 7.5gm of Sodium hydroxide (Solution B) • Warm the solution up to 600 C • Add pinch of erioglaucine to the Solution A. Use small quantity of dye sufficient to make solution blue. • Add solution B to A. Addition of solution results in change of colour from dark blue to light blue. • Pour this solution from the height of 60 cm in to empty beaker. Vigorous pouring from the height is essential to dissolve oxygen from air into the solution. The colour of the solution turns from light blue to dark blue.. • This experiment may be repeated numerous times. |
• Take 15 gm of benzoic acid as reducing agent in 750ml of distilled water (Solution A). • Take 250 ml of distilled water and add 7.5gm of Sodium hydroxide (Solution B) • Warm the solution up to 600 C • Add pinch of tetrazolium chloride to the Solution A. Use small quantity of dye sufficient to make solution red. • Add solution B to A. Addition of solution results in change of colour from dark red to light red. • Pour this solution from the height of 60 cm in to empty beaker. Vigorous pouring from the height is essential to dissolve oxygen from air into the solution. The colour of the solution turns from light red to dark red.. • This experiment may be repeated numerous times. |
|
Dye 1: Methylene Blue |
Dye 2: Indio Carmine (Colour Change reaction) |
|
• Take 15 gm of ascorbic as reducing agent in 750ml of distilled water (Solution A). • Take 250 ml of distilled water and add 7.5gm of Sodium hydroxide (Solution B) • Warm the solution up to 600 C • Add pinch of Methylene Blue to the Solution A. Use small quantity of dye sufficient to make solution blue. • Add solution B to A. Addition of solution results in change of colour from blue to colourless solution • Pour this solution from the height of 60 cm in to empty beaker. Vigorous pouring from the height is essential to dissolve oxygen from air into the solution. The colour of the solution turns blue again. • This experiment may be repeated numerous times. |
• Take 15 gm of ascorbic as reducing agent in 750ml of distilled water (Solution A). • Take 250 ml of distilled water and add 7.5gm of Sodium hydroxide (Solution B) • Warm the solution up to 600 C • Add pinch of Indio carmine (disodium salt of Indio 5-5- dysphonic acid) to the Solution A. Use small quantity of dye sufficient to make solution blue. • Add solution B to A. Addition of solution results in change of colour from blue to green and over time the green colour change to red/golden yellow. • Pour this solution from the height of 60 cm in to empty beaker. Vigorous pouring from the height is essential to dissolve oxygen from air into the solution. The colour of the solution turns green. • Once again, the colour of the solution turns into red/golden yellow. This experiment may be repeated numerous times. |
|
Dye 3: Safrene |
Dye 4: resazurin |
|
• Take 15 gm of ascorbic acid as reducing agent in 750ml of distilled water (Solution A). • Take 250 ml of distilled water and add 7.5gm of Sodium hydroxide (Solution B) • Warm the solution up to 600 C • Add pinch of safrene to the Solution A. Use small quantity of dye sufficient to make solution pink. • Add solution B to A. Addition of solution results in change of colour from dark pink to light pink. • Pour this solution from the height of 60 cm in to empty beaker. Vigorous pouring from the height is essential to dissolve oxygen from air into the solution. The colour of the solution turns from light pink to dark pink. • This experiment may be repeated numerous times. |
• Take 15 gm of ascorbic acid as reducing agent in 750ml of distilled water (Solution A). • Take 250 ml of distilled water and add 7.5gm of Sodium hydroxide (Solution B) • Warm the solution up to 600 C • Add pinch of resazurin to the Solution A. Use small quantity of dye sufficient to make solution pink. • Add solution B to A. Addition of solution results in change of colour from dark pink to light pink. • Pour this solution from the height of 60 cm in to empty beaker. Vigorous pouring from the height is essential to dissolve oxygen from air into the solution. The colour of the solution turns from light pink to dark pink. • This experiment may be repeated numerous times. |
|
Dye 5: erioglaucine |
Dye 6: Tetrazolium Chloride |
|
• Take 15 gm of ascorbic acid as reducing agent in 750ml of distilled water (Solution A). • Take 250 ml of distilled water and add 7.5gm of Sodium hydroxide (Solution B) • Warm the solution up to 600 C • Add pinch of erioglaucine to the Solution A. Use small quantity of dye sufficient to make solution blue. • Add solution B to A. Addition of solution results in change of colour from dark blue to light blue. • Pour this solution from the height of 60 cm in to empty beaker. Vigorous pouring from the height is essential to dissolve oxygen from air into the solution. The colour of the solution turns from light blue to dark blue.. • This experiment may be repeated numerous times. |
• Take 15 gm of ascorbic acid as reducing agent in 750ml of distilled water (Solution A). • Take 250 ml of distilled water and add 7.5gm of Sodium hydroxide (Solution B) • Warm the solution up to 600 C • Add pinch of tetrazolium chloride to the Solution A. Use small quantity of dye sufficient to make solution red. • Add solution B to A. Addition of solution results in change of colour from dark red to light red. • Pour this solution from the height of 60 cm in to empty beaker. Vigorous pouring from the height is essential to dissolve oxygen from air into the solution. The colour of the solution turns from light red to dark red.. • This experiment may be repeated numerous times. |

Objectives
-
To develop lesson plan by adopting experiential learning activities to acquire intended specific learning outcome.
-
To investigate the effectiveness of experiential learning activity among senior secondary school students in learning chemical redox reaction
-
To investigate the retention of learning redox reaction using experiential learning activity among Grade XII students.
Research Questions
-
Does Chemistry teachers have interest in active use of experiential learning activities to enable teaching-learning through innovation, creativity, production and high order thinking skills approach?
-
Has sustainable curriculum been constructed to foster, to catalyze and to improve learning through innovative and creative teaching-learning?
-
Does lesson plan integrate with the experiential learning active to enhance the effectiveness of teaching and deep conceptual understanding?
-
Do chemistry teachers use blended learning approach to cultivate critical thinking among learners? Does theoretical knowledge is applied with practical knowledge?
Hypothesis
-
There is noteworthy difference in the mean score in learning the concept Redox reaction between the tests before and after the treatment of learners of control group.
-
There will be a noteworthy variance in the mean score in learning the Redox reaction between pre-test and retention test of learners of control group.
-
There will be noteworthy difference in the mean score in learning the concept of redox reaction between post-test and retention test of learners of control group.
-
There will be a noteworthy difference in the mean score in learning the concept Redox reaction between the test before and after the treatment of learners of study group.
-
There will be a noteworthy variance in the mean score in learning the concept of Redox reaction between pre-test and retention test of learners of study group.
-
There will be noteworthy difference in the mean score in learning the concept of redox reaction between post-test and retention test of learners of study group.
-
There will be noteworthy difference in the gain score in learning the concept of redox reaction among the control group and study group.
-
There will be noteworthy difference in the gain score in retention of learning the concept of redox reaction between study group and control group learners.
METHODS
RESEARCH DESIGN
In the present paper quasi-experimental design is applied.
POPULATION
All students of Grade XI from the study area.
SAMPLE
Totally 50 students took part in the study. To draw the sample stratified random sampling method is applied. Sample is categorized into two groups control group and the study group. Each group consists of 25 students. Students of the study group are taught the concept of Redox reaction using experiential learning activities and the control group is taught by lecture method. The treatment lasts for 30 days.
INSTRUMENT
Data is collected by using formative assessment before and after the treatment and retention test after the treatment. Test is validated by experts.
RESULT AND DISCUSSIONS
H 0 1: There is noteworthy difference in the mean score in learning the concept Redox reaction between the tests before and after the treatment of learners of control group.
|
Variables |
Test |
N |
Mean |
Mean Diff |
SD |
Df |
t-var |
p-var |
|
Specific Learning Outcome |
Pre-test |
25 |
348 |
5.25 |
1.05 |
24 |
14.50 |
.000 |
|
|
Post-test |
|
8.73 |
|
8.73 |
|
|
|
H 0 2: There will be a noteworthy variance in the mean score in learning the Redox reaction between pre-test and retention test of learners of control group.
|
Variables |
Test |
N |
Mean |
Mean Diff |
SD |
Df |
t-var |
p-var |
|
Specific Learning Outcome |
Pre-test |
25 |
348 |
8.3 |
1.05 |
24 |
21.15 |
.000 |
|
|
Retention test |
|
11.78 |
|
1.32 |
|
|
|
H 0 3: There will be noteworthy difference in the mean score in learning the concept of redox reaction between post-test and retention test of learners of control group.
|
Variables |
Test |
N |
Mean |
Mean Diff |
SD |
Df |
t-var |
p-var |
|
Specific Learning Outcome |
Post-test |
25 |
8.73 |
3.50 |
1.41 |
24 |
15.80 |
.000 |
|
|
Retention test |
|
11.78 |
|
1.49 |
|
|
|
H 0 4: There will be a noteworthy difference in the mean score in learning the concept Redox reaction between the test before and after the treatment of learners of study group.
|
Variables |
Test |
N |
Mean |
Mean Diff |
SD |
Df |
t-var |
p-var |
|
Specific Learning Outcome |
Pre-test |
25 |
4.72 |
8.85 |
1.69 |
24 |
28.57 |
.000 |
|
Post test |
13.57 |
1.10 |
H 0 5: There will be a noteworthy variance in the mean score in learning the concept of Redox reaction between pre-test and retention test of learners of study group.
|
Variables |
Test |
N |
Mean |
Mean Diff |
SD |
Df |
t-var |
p-var |
|
Specific Learning Outcome |
Pre-test |
25 |
4.72 |
12.14 |
1.89 |
24 |
14.84 |
.000 |
|
|
Retention test |
|
16.86 |
|
1.20 |
|
|
|
H 0 6: There will be noteworthy difference in the mean score in learning the concept of redox reaction between post-test and retention test of learners of study group.
|
Variables |
Test |
N |
Mean |
Mean Diff |
SD |
Df |
t-var |
p-var |
|
Specific Learning Outcome |
Post-test |
25 |
13.57 |
3.29 |
1.79 |
24 |
14.84 |
.000 |
|
|
Retention test |
|
16.86 |
|
1.20 |
|
|
|
H 0 7 : There will be noteworthy difference in the gain score in learning the concept of redox reaction among the control group and study group.
|
Variables |
Test |
N |
Mean |
Mean Diff |
SD |
Df |
t-var |
p-var |
|
Specific Learning Outcome |
Post-test |
25 |
4.95 |
4.58 |
2.70 |
24 |
6.85 |
.000 |
|
|
Retention test |
|
9.53 |
|
1.51 |
|
|
|
H 0 8: There will be noteworthy difference in the gain score in retention of learning the concept of redox reaction between study group and control group learners.
|
Variables |
Test |
N |
Mean |
Mean Diff |
SD |
Df |
t-var |
p-var |
|
Specific Learning Outcome |
Post-test |
25 |
11.78 |
5.08 |
1.20 |
24 |
12.68 |
.000 |
|
|
Retention test |
|
16.86 |
|
1.53 |
|
|
|
According to the result illustrated in Table 4 calculated t-value is statistically noteworthy at 0.001 level and hence hypothesis “There will be a noteworthy difference in the mean score in learning the concept Redox reaction between pre-test and post-test of learners of control group” is accepted. The mean score of the post-test is higher than the pre-test, when the unit is taught by using conventional chalk and talk method.
Result of Table 5 reveals that mean score of retention test is higher than pre-test score. The calculated t-value is statistically noteworthy at 0.001 level and hence hypothesis “There will be a noteworthy difference in the mean score in learning the concept of Redox reaction between pre-test and retention test of learners of control group” is accepted. Result of the present paper reveals that the conventional chalk-board method foster learning among students and retain the same.
Result of Table 6 reveals that the mean score of retention is higher than the post-test due to manipulation of concept in conventional blackboard teaching-learning after post-test. The calculated t-value is statistically noteworthy at 0.001level. Hence hypotheses “There will be noteworthy difference in the mean score in learning the concept of redox reaction between the test before the treatment and retention test of learners of control group” is accepted.
Result in Table 7 illustrates that the score of post-test is higher than the pre-test of the study group students when exposed to experiential learning activity when learning redox reaction. It may be concluded that experiential learning activity fosters conceptual understanding among students of study group. The calculated t value is statistically noteworthy at 0.001 level and hence the null hypothesis “There will be noteworthy difference in the mean score in learning the concept Redox reaction between the tests before and after the treatment of learners of study group” is accepted.
Result of Table 8 reveals that the mean score in the retention test is greater than the pre-test of the students in study group. The experiential learning activity fosters retention of learning. The t-value is statistically noteworthy at 0.001 level. Hence the hypothesis “There will be noteworthy difference in the mean score in learning the concept of Redox reaction between pre-test and retention test of learners of study group” is accepted.
It is noticed from Table 9 that the retention mean score is than the mean score of post-test of the study group due to the manipulation of the concept through experiential learning activity and reinforcement in teaching-learning process. The t-value is statically noteworthy at 0.001 level. The null hypothesis “There will be noteworthy difference in the mean score in learning the concept of redox reaction between post-test and retention test of learners of study group” is accepted.
It is evident from Table 10 that the students in the study group score higher than their counterparts. Hence it is concluded that experiential learning activity is better for teaching-learning compared to the conventional method. The calculated t-value is statistically noteworthy at 0.001 level. Hence the hypothesis “There will be noteworthy difference in the gain score in learning the concept of redox reaction among the control group and study group” is accepted.
Result of Table 11 illustrates that the gain score of the retention test of students from study group is higher than the students from the control group. The calculated t-value is statistically noteworthy at 0.001 level. Hence hypothesis “There will be noteworthy difference in the gain score in learning the concept of redox reaction among the control group and study group” is accepted.
There is noteworthy difference in the mean score between two groups in the test before the treatment, post-test and retention test om attaining the specific learning outcomes. Retention of the concept of redox reaction among the students of study group is more when compared to the control group.
DISCUSSION
Teachers develop more appropriate learning opportunities for the targeted students to attain the specific learning outcome. Experiential learning activities enable students to go through each and every stage of learning cycle. Learning through experiential learning activity is conceived only as a process and not in terms of attainment of specific learning outcomes. Learning is incomplete when the attainment of specific learning outcome is not evident in performance. Learning takes place during the passage of connected experience in which knowledge is reformed and modified. Education is continuous reconstruction of experience.
All learning is re-learning. Hence in the present study experiential learning activity with various reducing agents such as ascorbic acid and benzoic acid and various dyes such as Methylene blue, Indio Carmine, safrene, resazurin, erioglauncine, tetrazolium Chloride are used to repeat the activity. Learning is well facilitated by these processes and it draws out the students’ ideas and beliefs about the concept, so that it may be tested, examined and amalgamated with more refined ideas. Learners construct their knowledge of world on the basis of their gained experience.
Learning is a holistic process of adoption. Learning is the result of not only cognitive development but it also involves integrated functioning of total person – thinking, feeling, perceiving and behaving. It fosters scientific methods for problem solving, decision making and creativity. Learning results from the synergetic transaction between students and environment. Hence learning is the process of creating knowledge.
CONCLUSION
Learning takes place in a positive and motivating environment. Learners have better self-esteem and learn positively from their peer groups and their ecosystem. In world where, pressure and stress in everyday life are increasing affecting the mental status of both students and teachers, many times traditional methods are not adequate. The society should take initiative for making the school a happy place and making learning a joyful experience.
Joyful learning experience and happy school ecosystem fosters holistic development of the learners. Experiential learning makes educational climate in school as child-centered. The learners are directed towards their learning. It enhances co-operation, collaboration and team work. Experiential learning activity catalyzes learning, provides safe environment, links theory and practice, demonstrates change and enhances participation in learning and retention.

