Effect of Brazil nut (Bertholletia excelsa) supplementation on human health: a systematic review
Abstract
Previous studies suggest that Brazil nut can improve the nutritional status of selenium, reduce oxidative stress, modify lipid profile, and alter inflammatory markers. A systematic review was performed to evaluate and synthesize the results of studies on the effect of Brazil nut supplementation on human health. The review was conducted according to the recommendations of the Preferred Reporting Items for reviews and was based on searches of the databases BIREME, BMC Proceedings, The British Library, The Cochrane Library, and PubMed up to December 2019. The searches also included at the databases Google Scholar and Web of Sciences. The descriptors were identified using the Health Science Descriptors system and the search strategy included the following combination of keywords: Bertholletia, protein, lipid, nutritive value, and selenium; in English and Portuguese. A total of 510 potential studies were screened, of which 25 studies were considered for this review. All studies showed that supplementation with Brazil nut in the amounts evaluated improved the selenium status in the body and had positive effects without reports of adverse effects. This systematic review indicates that Brazil nut has a beneficial effect on human health, but further studies with double-blind controlled clinical trials and larger sample sizes are needed for the validation of these effects.
Keywords
Selenium, Lipid Profile, Oxidative Stress, Supplementation
INTRODUCTION
Brazil nut (Bertholletia excelsa) is a typical tree of the Amazon Forest (northern or northeastern Brazil). It belongs to the Lecythidaceae family and produces fruits with high nutritive value, containing 15 to 20% protein and 60-70% lipids, as well as antioxidant properties due to its content of vitamin E and Selenium (Se). Brazil nuts have a high caloric content mainly because of its levels of saturated, monounsaturated, and polyunsaturated fatty acids in the proportion of 25:41:34, respectively. They are also high in vital minerals such as calcium, magnesium, phosphorus, potassium, and selenium (Ariane et al., 2015; Ryan, Galvin, O'Connor, Maguire, & O'Brien, 2006).
Selenium is an essential micronutrient for human health. Its biological functions are mediated by the expression of approximately 20 selenoproteins that contain selenocysteine in their active sites. Adequate Se intake is required for the normal activity of numerous selenoenzymes involved in protection against oxidative stress, immune system regulation, and thyroid function (Ariane et al., 2015).
Bovine kidney and liver, seafood, fish, meat, and wheat are good sources of selenium. However, it is of note that the amount of selenium contained in food reflects its concentrations in the soil and, therefore, the same type of food may have a different concentration of the mineral in different soils (Rayman, 2008). Brazil nuts contain large concentrations of selenium, ranging between 5.8 and 169.9 μg/g of the nutrient, thus constituting an important source of the element (Ariane et al., 2015; Martins et al., 2012). The daily recommendation for adults aged 19 or more is 55 μg/day (Otten, Hellwig, Meyers, & Dri, 2006).
Previous studies have suggested that Brazil nut can improve the nutritional status of selenium, reduce oxidative stress, modify lipid profile, and alter inflammatory markers in the human body (Ariane et al., 2015; Rayman, 2008; Ryan et al., 2006). Therefore, this systematic review will provide a synthesis of reports that investigated the effect of Brazil nut supplementation on human health.
METHOD
DATABASE AND STUDY OF ELIGIBILITY
The systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Liberati et al., 2009). The defined research question was "What is the beneficial effect of Brazil nut (Bertholletia excelsa) supplementation on human health?"
Five databases, including BIREME (Latin American and Caribbean Center for Health Sciences Information), BMC Proceedings (BioMed Central), The British Library, The Cochrane Library, and PubMed were searched from October to December 2017. In addition to these databases, a search was performed at Google Scholar, and the first 60 articles that appeared in order of relevance were included. A further search was carried out with Web of Science for relevant articles published in 2018 and 2019. The descriptors Bertholletia, protein, lipid, nutritive value, and selenium were identified using the Health Science Descriptors (DeCS), in both English and Portuguese languages, and combined in the search strategy.
The studies considered eligible for this review involved clinical trials with humans, who received Brazil nut supplementation, without age limits. Studies involving animals and not related to human health, reviews, case studies, and theses were excluded.
DATA EXTRACTION
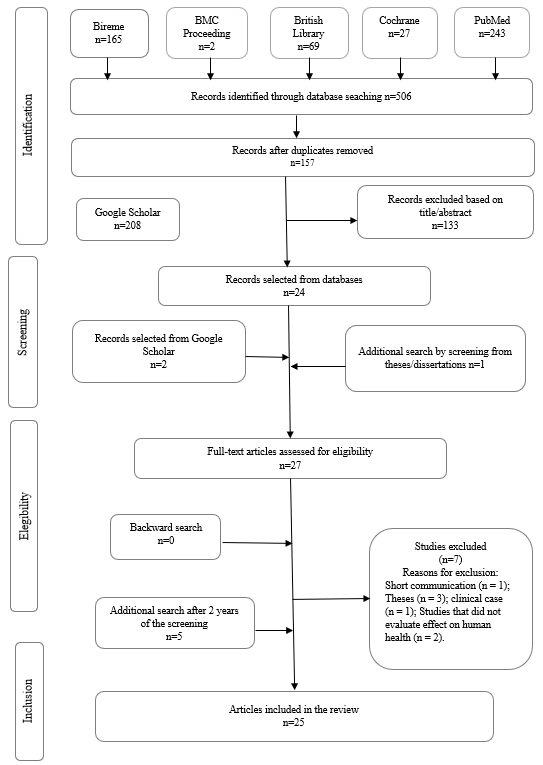
From the 506 potential studies identified by the keywords, 25 were selected for reading, and 21 were included in the review (Figure 1 ), and another 4 studies were included after the first screening. The screening was performed in 2 steps. In the first step, three researchers (DCR, KPC, and HSM) independently read the titles and abstracts of all records identified and excluded the duplicates and those that did not meet the eligibility criteria. In the second step, the three researchers read the papers selected in the first step independently, and the same inclusion criteria were applied to confirm their eligibility. The disagreements were resolved via consensus or through consultation with the coordinator (DCR).
The methodological quality of the studies was assessed by the rating scale proposed by Downs and Black (1998) (Downs & Black, 1998). The Downs and Black Scale consists of 27 questions relating to the quality of reporting (clarity), external validity (representativeness), internal validity (bias and confounding), and statistical power of the study. The 27 questions of the scale were responded, and the responses were assigned a grade "1" (when the criterion that characterized quality was present) or "0" (when the criterion was absent). The modified scale rates the quality of the study using grades from zero (poor) to 27 (excellent).
RESULTS
The data extracted from each study included reference details, year of publication, sample, and study characteristics, age, sex, objective, types of intervention, and results (Table 1 ). Of the 25 studies included in this review, 23 were conducted in Brazil, 1 in Australia, and 1 in New Zealand. The smallest sample size was 10 subjects, and the largest sample size was 129. Of the 21 studies, 9 involved healthy volunteers. Two studies had participants with mild cognitive impairment; 6 studies had participants under dialytic treatment, and the other 14 studies had participants with chronic noncommunicable diseases such as obesity, hypertension, and dyslipidemia. Ages ranged from 2 to 80 years, and 70 % of them were female.
|
Study Design |
Sample and Characteristics |
Age (years) |
Sex (%) |
Intervention |
Results |
Reference/Country |
||
|
RCI |
20 adults with mild cognitive impairment |
≥60 |
70 % women |
1 BN a day for 6 months. Se serum concentrations, erythrocyte GPx activity, oxygen radical absorbance capacity, and malondialdehyde radicals were measured before and after intervention. Cognitive function was assessed using the CERAD battery. |
BN intake can promote recovery of SE deficiency and positive effects on verbal fluency and cognitive praxis. |
Cardoso et al. 2015 [ (Cominetti, Bortoli, Garrido, & Cozzolino, 2012) ] Brasil |
||
|
RCI |
20 older adults with mild cognitive impairment |
≥60 |
70 % women |
1 BN a day for 6 months. Dosage of GPx activity, SE serum levels, selenoprotein rs1050450, rs7579 and rs3877899 genotyping, and expression of GPX1 and SEPP gene were performed. Intake of SE was assessed through a 3-day food recall. |
GPX1 and rs1050450 polymorphism can influence SE status and selenoprotein synthesis. GPX1 and SEPP expression in response to BN ingestion was affected by rs1050450, rs7579 and rs3877899 genotypes, but did not directly alter SE status. |
Cardoso et al. 2016[ (Donadio, Rogero, Cockell, Hesketh, & Cozzolino, 2017) ] Brasil |
||
|
CI |
25 patients on hemodialysis |
≥18 |
64 % women |
1 BN a day for 3 months. Biochemical parameters, oxidative stress and inflammation markers were measured in fasting subjects before and after the dialysis session baseline and after 3 months of intervention. qCRP analysis was done for expression of Nrf2. |
BN supplementation activated Nrf2 in HD patients and may possibly explain the beneficial effects. |
Cardozo et al. 2016 [ (Donadio et al., 2018) ] Brasil |
||
|
RCI |
77 hypertensive and dyslipidemic patients |
40 to 80 |
44.2 % women |
13 g of BN daily (defatted flour) for 3 months. Thyroid hormones, TG, TC, and HDL were measured before the study and monthly during 3 months. |
Reduction in TC, but not in HDL. No change in thyroid hormones. |
Carvalho et al. 2015[ (Donadio et al., 2019) ] Brasil |
||
|
RCS |
10 healthy volunteers |
23 to 34 |
40 % women |
Doses of 0, 5g, 20g, and 50g of BN once. At each intervention, TC, HDL, LDL, TG, SE, liver enzymes, albumin, total proteins, renal function, and CRP were measured at 1,3,6,9,24, and 48 hours and on days 5 and 30.. |
Improvement in the lipid profile with a BN portion of only 5 g. High BN intake did not produce hepatic and renal toxicity. |
Colpo et al. 2013[ (Duarte et al., 2019) ] Brasil |
||
|
RCS |
10 healthy volunteers |
23 to 34 |
40 % women |
Doses 0, 5g, 20g, and 50g of BN once. Interleukins 1.6 and 10, tumor necrosis factor, interferon, hepatic enzymes, albumin, total protein, and renal function were measured at 1,3,6,9,24, and 48 hours and on days 5 and 30. |
Single intake of 20 to 50 g of BN decreased inflammatory markers up to 30 days after ingestion. |
Colpo et al. 2014[ (Hu et al., 2016) ] Brasil |
||
|
UCI |
37 morbidly obese premenopausal women |
≥18 |
100 % women |
1 BN a day for 8 weeks. Serum SE concentration and erythrocyte GPx activity were measured at the baseline and after the study. DNA extraction and genotyping and measurement of the level of DNA damage were performed. |
Improvement in SE status and increase in GPx activity irrespective of the GPX1 Pro198Leu polymorphism. Regarding the level of DNA damage, individuals with the homozygous Leu/Leu genotype showed higher levels after supplementation that may influence the risk of non-communicable chronic diseases. |
Cominetti et al. 2011[ (Huguenin et al., 2015) ] Brazil |
||
|
UCI |
37 morbidly obese premenopausal women |
≥18 |
100 % women |
1 BN a day for 8 weeks. The serum SE concentration and the GPx activity were measured, and the lipid profile assessed at the baseline and after the study. The risk of atherogenesis was also determined. |
Significant increase in HDL lipoprotein, improving the lipid profile and reducing the risk of atherogenesis. |
Cominetti et al. 2012[ (Huguenin et al., 2015) ] Brazil |
||
|
CI |
130 healthy volunteers |
20 to 60 |
75 % women |
1 BN a day for 8 weeks. Gene expression and genotyping of GPX1 and SELENOP were performed. |
Significant increase of GPX1 mRNA expression only in subjects with CC genotype at rs1050450. SELENOP mRNA expression was significantly higher in A-carriers at rs7579 either before or after supplementation. Genotype for rs713041 in GPX4 affected the pattern of blood cell global gene expression. Genetic variations in selenoprotein genes modulated both GPX1 and SELENOP selenoprotein gene expression and global gene expression. |
Donadio et al. 2017[ (Maranhão et al., 2011) ] Brazil |
||
|
CI |
130 healthy volunteers |
20 to 60 |
75 % women |
1 BN a day for 8 weeks. Anthropometric measurements, serum fasting glucose, lipid profile, CRP, plasma Malondialdehyde Levels were performed. The volunteers were genotyped for SNPs rs1050450, rs3811699, rs1800699, rs713041, rs3877899, rs7579, rs34713741, and rs5845 in genes for SELENOP. |
Reduced total cholesterol and glucose levels, suggesting that rs3877899 might be associated with glucose concentrations and rs7579 with cholesterol concentrations. |
Donadio et al. 2018[ (Martens et al., 2015) ] Brazil |
||
|
CI |
130 healthy volunteers |
20 to 60 |
75 % women |
1 BN a day for 8 weeks, followed by 8 weeks of washout. Blood samples were collected at baseline, 4 and 8 weeks of supplementation, and 4 and 8 weeks of washout for analysis of five biomarkers of Se status. The gene expression was quantified before and after 8 weeks of supplementation. |
Four biomarkers of Se status increased significantly after 4 and 8 weeks of supplementation, affected by gender. Erythrocyte GPx1 activity was associated with rs1050450, rs713041 and rs5845. Plasma SE was associated with rs7579 and SEPP with plasma SE at baseline. Supplementation significantly increased GPX1 mRNA expression only in subjects with CC genotype at rs1050450. SELENOP mRNA expression was significantly lower in subjects with GG genotype at rs7579. |
Donadio et al. 2019[ (Reis et al., 2019) ] Brazil |
||
|
RCI |
55 obese women |
18 to 55 |
100% women |
1 BN a day for 2 months. At baseline and after 2 months, analyses of biochemical parameters related to SE status, oxidative stress, and inflammatory biomarkers were performed. |
No changes in plasma inflammatory biomarkers levels, but a significant increase in gene expression that may indicate a proinflammatory stimulus in obesity induced by the consumption of BN with high SE levels. |
Duarte et al. 2019[ (Stockler-Pinto, Mafra, Farage, Boaventura, & Cozzolino, 2010) ] Brazil |
||
|
RCI |
32 healthy volunteers |
> 50 |
54 % women |
6 BN and 4 GTE capsules were administered, alone and combined, for 6 weeks. Biochemical exams and rectal biopsy were performed before and after the intervention. |
Reduction of the risk of colorectal cancer regulated by selenoprotein-associated genes; the combination had no additional effects. |
Hu et al. 2016[ (Stockler-Pinto et al., 2012) ] Austrália |
||
|
RCCI |
91 hypertensive and dyslipidemic patients |
62 ± 9.3 |
48.4 % women |
13g of granulated and partially defatted BN for 3 months. Biochemical tests and evaluation of microvascular endothelial function were performed before and after the intervention. |
Increase in plasma SE concentration; no improvement in endothelial microvascular function. |
Huguenin et al. 2015a[ (Stockler-Pinto et al., 2014) ] Brazil |
||
|
RCCI |
91 hypertensive and dyslipidemic patients |
62 ± 9.3 |
51.6 % men |
13g of granulated and partially defatted BN for 3 months. Plasma SE, GPx3 activity, total antioxidant capacity, 8-epi PG, and oxidized LDL were evaluated before and after the intervention |
Increase in plasma SE concentration and in GPx3 activity, and a reduction in LDL oxidation. |
Huguenin et al., 2015b[ (Stockler-Pinto et al., 2015) ] Brazil |
||
|
RCI |
17 obese adolescents |
15.4 ± 2 |
100 % women |
3 to 5 BN daily for 16 weeks. Anthropometry, lipid-metabolic profile, oxidative stress, diameters and capillary function through videocapilaroscopy were evaluated. |
No change in body mass or waist circumference, but a positive effect on lipid profile and nutritional skin microcirculation. |
Maranhão et al. 2011[ (Stockler-Pinto et al., ) ] Brazil |
||
|
CI |
129 preschoolers |
2 to 6 |
50 % women |
3 BN, 3 times a week for 7 months. SE status was analyzed through the plasma, erythrocyte, nail, hair and urine dosages. |
Increase in the levels of SE and an excessive intake of the mineral. |
Martens et al. 2015[ (Strunz et al., 2008) ] Brazil |
||
|
RCI |
54 obese women |
18 to 55 |
100% women |
1 BN a day for 2 months. Biochemical parameters related to SE status and 25 circulating miRNAs in plasma were evaluated at baseline and after 2 months. |
Increase in circulating miR-454-3p and miR-584-5p expression levels in obese women. |
Reis et al. 2019[ (Thomson, Chisholm, McLachlan, & Campbell, 2008) ] Brazil |
||
|
UCI |
81 patients on hemodialysis |
52 ± 16.1 |
32.1 % women |
1 BN a day, for 3 months. SE serum levels and GPx activity were measured before and after supplementation. |
SE nutritional status of patients improved. Significant increase in GPx levels. |
Stockler-Pinto et al. 2010[ (Ariane et al., 2015) ] Brazil |
||
|
UCI |
21 patients on hemodialysis |
54.2 ± 15.2 |
32.1 % women |
1 BN a day, for 3 months. Se serum levels were measured after 12 months. |
SE levels were not as low as pre-supplementation levels. |
Stockler-Pinto et al. 2012[ (Ryan et al., 2006) ] Brazil |
||
|
UCI |
40 patients on hemodialysis |
53 ± 16.1 |
57.5 % women |
1 BN a day, for 3 months. The activity of GPx, 8-isoprostane, 8-OHdG, and cytokine levels (TNF-α and IL-6) and lipid profiles were measured before and after. |
Increase in GPx and HDL levels, cytokines, deoxyguanosine, and 8-isoprostane, 8-OHdG and LDL decreased significantly. |
Stockler-Pinto et al. 2014[ (Rayman, 2008) ] Brazil |
||
|
UCI |
21 patients on hemodialysis |
51 ± 3.3 |
41.4 % women |
1 BN a day for 3 months. Antioxidants, SE, GPx, 8-Isoprostane, 8-OHdG and cytokines (TNF-α and IL-6) were measured before and after 3 and 12 months. |
After 12 months, levels of 8-isoprostane, 8-OHdG, and cytokine increased and plasma SE and GPx activity decreased significantly. |
Stockler-Pinto et al. 2015a[ (Martins et al., 2012) ] Brazil |
||
|
UCI |
40 patients on hemodialysis |
53 ± 16.1 |
57.5 % women |
1 BN a day for 3 months. Serum levels of SE, GPx activity and hormones, TSH, T3 and T4 were measured before and after. |
Increase in plasma SE levels, of the GPx activity, and T3 and T4 levels. |
Stockler-Pinto et al. 2015b[ (Otten et al., 2006) ] Brazil |
||
|
UCI |
15 healthy volunteers |
27.3 ± 3.9 |
66.6 % women |
45 g of BN a day for 15 days. HDL particle size, paroxytone 1 activity, and lipid transfer from a lipoprotein-like nanoparticle to the HDL fraction were analyzed. |
No alteration of the lipid profile. Regarding the functional properties of HDL, increase in cholesterol ester receptors by the lipoprotein, which may be a protective mechanism of atherosclerosis. |
Strunz et al. 2008[ (Liberati et al., 2009) ] Brazil |
||
|
CI |
59 healthy adults |
18 a 60 |
48 % women |
2 BN a day for 12 weeks. SE serum levels and GPx activity were measured at 0, 2, 4, 8, and 12 weeks. |
BN was as effective as selenomethionine in improving SE status and GPx activity. |
Thomson et al. 2008[ (Downs et al., 1998) ] New Zealand |
||
Participants received between one and six Brazil nuts a day, and supplementation was given from 15 days to 7 months. In two studies that evaluated the effect of moderate intake, participants received up to 50 g, but in a single dose, corresponding to approximately 10 medium-sized nuts. None of these studies has reported any adverse effects from the use of Brazil nut. The Downs and Black scale (1998) assigned the lowest score of 11 to 4 articles (19%) and the highest score of 22 to 2 articles (9.5%), indicating the methodological quality (Table 2 ). The best-evaluated criteria were quality of reporting and internal validity, and only two studies showed statistical power.
|
Criterion Score (%) |
|
|
Reporting |
|
|
1. Objective/hypothesis clearly described ______________________________________ |
100% |
|
2. Main outcomes to be measured clearly described in the Methods section ___________ |
100% |
|
3. Characteristics of patients clearly described __________________________________ |
96% |
|
4. Interventions of interest clearly described ____________________________________ |
95,2% |
|
5. Confounders in each group of subjects to be compared clearly described____________ |
0% |
|
6. Main findings of the study clearly described ___________________________________ |
100% |
|
7. Study provides estimates of the random variability in the data for the main outcomes___ |
100% |
|
8. All important adverse events resulted from the intervention have been reported_______ |
0% |
|
9. Characteristics of patients lost to follow-up have been described___________________ |
35% |
|
10. Actual probability values have been fully reported ______________________________ |
42,8% |
|
External Validity |
|
|
11. Representatives of the entire population from which they were recruited______________ |
9,5% |
|
12. Representatives of the specific population from which they were recruited ____________ |
0% |
|
13. Staff, places, and facilities where the patients where treated were representative________ |
61,9% |
|
Internal Validity - bias |
|
|
14. Attempt was made to blind study subjects to the intervention they have received _______ |
14,3% |
|
15. Attempt was made to blind those measuring the main outcomes of the intervention _____ |
19% |
|
16. It was made clear whether results of the study were based on “data dredging”_________ |
0% |
|
17. Time between the intervention and outcome was the same for cases and controls______ |
100% |
|
18. Statistical tests used to assess the main outcomes were appropriate ________________ |
95,2% |
|
19. Compliance with the intervention/s was/were reliable ____________________________ |
90,5% |
|
20. Main outcome measures used were valid and reliable ___________________________ |
100% |
|
Internal Validity - confounding |
|
|
21. Patients were recruited from the same population ______________________________ |
95,2% |
|
22. Study subjects were recruited over the same period of time _______________________ |
28,6% |
|
23. Study subjects were randomized to intervention groups __________________________ |
52,4% |
|
24. Intervention was concealed from both patients and health care staff ________________ |
23,8% |
|
25. There was adequate adjustment for confounding in the analyses __________________ |
14,3% |
|
26. Losses of patients to follow-up were taken into account __________________________ |
28,6% |
|
Power of Study 27. The study have sufficient power to detect a clinically important effect _______________ |
9,5% |
|
Total = 25 studies |
|
DISCUSSION
All studies showed that Brazil nut supplementation, in the amounts and time assessed, improved the selenium status without any adverse effects (Cardoso et al., 2016; Cardoso et al., 2016; Cardozo, Stockler-Pinto, & Mafra, 2016; Carvalho et al., 2015; Colpo et al., 2013; Colpo et al., 2014; Cominetti et al., 2011; Cominetti et al., 2012; Donadio et al., 2018; Donadio et al., 2019; Donadio et al., 2017; Downs et al., 1998; Duarte et al., 2019; Hu et al., 2016; Huguenin et al., 2015; Huguenin et al., 2015; Liberati et al., 2009; Maranhão et al., 2011; Martens et al., 2015; Reis et al., 2019; Stockler-Pinto et al., 2012; Stockler-Pinto et al., 2014; Stockler-Pinto et al., 2010). It is of note that the minimum amount used was one nut a day, which is equivalent to approximately 288.75 μg selenium. The daily requirement for adults aged 19 years and over is 55 μg/day, and tolerable upper intake level (UL) 400 μg/day. Thus, studies have shown that the constant ingestion of small doses of Brazil nut is enough to improve the selenium status, and with immediate effect, that is, from the first day. However, Cominetti et al. (Cominetti et al., 2011) found that individuals with the homozygous Leu/Leu genotype showed greater DNA damage after Brazil nut supplementation. Furthermore, Martens et al. (Huguenin et al., 2015) highlight in their study that despite the children were asymptomatic, they were at risk for toxicity.
The selenium concentration may vary significantly between different regions, harvests, and batches. One Brazil nut may contain up to 400 μg of selenium or more. This concentration may influence the response to different interventions (Stockler-Pinto et al., ; Stockler-Pinto et al., 2015). The brain is particularly vulnerable to oxidative stress because of its high rate of oxygen consumption and the presence of important amounts of unsaturated fatty acids that serve as substrates for lipid peroxidation. Antioxidant defenses can interfere with any of the three phases of the oxidative processes: initiation, propagation, or termination (Cardoso et al., 2016; Cardoso et al., 2016; Cardozo et al., 2016). Hence, much attention has been focused on minerals that play an essential role as constituents of antioxidant enzymes such as selenium. This mineral has an important role in antioxidant selenoproteins, with emphasis on the enzyme glutathione peroxidase and selenoprotein P, which are expressed abundantly in the brain. Selenoproteins may have an important role as antioxidants. Based on this premise, studies that evaluated cognitive function found that supplementation with Brazil nut can recover selenium deficiency, with positive effects on some cognitive functions such as verbal fluency and constructive praxis. These were preliminary studies, and further clinical studies with larger samples are required to confirm the results (Cardoso et al., 2016; Cardoso et al., 2016; Cardozo et al., 2016).
Studies on the regulation of thyroid hormones showed an increase in thyroid hormones in patients with low selenium levels and no alteration in euthyroid patients, indicating a possible role of Brazil nut in the regulation of these hormones, probably via selenium (Carvalho et al., 2015; Huguenin et al., 2015). There was an improvement in the lipid profile of both dyslipidemic and hypertensive patients, as well as healthy participants. It is important to highlight the isolated and combined effects of selenium and/or unsaturated fatty acids found in Brazil nut on atherogenic parameters. Further research is needed, however, to better understand the mechanisms of action in the modulation of cardiac indices and to establish whether the supplementation of dyslipidemic patients with Brazil nut would be a direction to follow for the treatment (Carvalho et al., 2015; Colpo et al., 2013; Cominetti et al., 2012; Donadio et al., 2019; Duarte et al., 2019; Hu et al., 2016; Stockler-Pinto et al., 2012).
Brazil nut intake has decreased inflammatory markers, which are biomarkers associated with the risk of colorectal cancer. It was also able to modulate the gene expression of some inflammatory markers such as Nrf2 (nuclear factor E2-related factor-2) and glutathione peroxidase, responsible for inflammation and oxidative stress. However, it was not clear whether the mechanism of action for the reduction of the inflammatory response would be via the gene expression of these markers (Cardozo et al., 2016; Colpo et al., 2014; Cominetti et al., 2011; Cominetti et al., 2012; Donadio et al., 2018; Donadio et al., 2017; Hu et al., 2016; Huguenin et al., 2015; Huguenin et al., 2015; Maranhão et al., 2011; Martens et al., 2015; Reis et al., 2019).
In conclusion, the present review corroborates literature assumptions that the Brazil nut has a beneficial effect on human health. Nevertheless, further studies with double-blind controlled clinical trials and larger sample size are needed for the validation of these effects.
ACKNOWLEDGMENTS
This study was supported by the Brazilian Funding Agencies CNPq (Conselho Nacional de Desenvolvimento Cientifico e Tecnológico) and Fapemig (Fundação de Amparo à Pesquisa do Estado de Minas Gerais). We thank the reviewers for constructive comments.
Register Number at PROSPERO: CRD420181070.

