
In- Vitro Antibacterial Activity of Spilanthes acmella (Akarkara) Extract on Porphyromonas gingivalis and Aggregatibactor actinomycetemcomitans
Dr. Renu Gaur 1![]()
![]() , Dr. Gazala MP
2
, Dr. Gazala MP
2![]()
![]() , Dr. Prabhuji MLV 3
, Dr. Prabhuji MLV 3![]()
![]()
1 Post
Graduate Student, Department of Periodontology, Krishnadevaraya
College of Dental Sciences, Bangalore, India
2 Senior
Lecturer, Department of Periodontology, Krishnadevaraya
College of Dental Sciences, Bangalore, India
3 Professor
and Head, Department of Periodontology, Krishnadevaraya
College of Dental Sciences, Bangalore, India
|
|
ABSTRACT |
||
|
Periodontal diseases are caused by certain bacteria found in the bacterial plaque. Usage of plant-derived antimicrobial agents could serve as an effective alternative treatment against periodontal infections due to continuous rise seen in antibiotic resistance. Spilanthes acmella (S. acmella), a vital medicinal plant has been used for its various properties such as anti- inflammatory, antibacterial, antifungal, antinociceptive, anti-cancerous and hastening wound healing. This study was conducted to assess its antibacterial efficacy against common periodontal pathogens. Objective: The present study was conducted to assess the antibacterial activity of S. acmella plant extract against Porphyromonas gingivalis (Pg), and Aggregatibactor actinomycetemcomitans (Aa) and determine the presence of various phytochemicals in it. Materials and Methodology: An extract was prepared using dried S. acmella plant powder and mixed with methanol in 1:1 (w/v) ratio. Determination of minimal inhibitory concentration (MIC) was done by using tube dilution technique and time- kill assay was performed against Pg and Aa. Presence of phytochemicals was checked by thin layer chromatography (TLC) method. Results: MIC of S. acmella was found to be 40 µg/ml for Pg and 20 µg/ml for Aa within 2 h interval. Various phytochemicals were found in S. acmella extract which may be responsible for its anti- bacterial property. Conclusion:
S. acmella extract
shows a significant antibacterial effect against the major periodontal
pathogens and hence may be a potential natural alternative for controlling
the growth of these bacteria. |
|||
|
Received 05 January 2024 Accepted 12 March 2024 Published 21 March 2024 Corresponding Author Dr. Renu
Gaur, renugaur497@gmail.com DOI 10.29121/jahim.v4.i1.2024.45 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2024 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Spilanthes Acmella, Porphyromonas Gingivalis, Aggregatibactor Actinomycetemcomitans, Minimal
Inhibitory Concentration, Time Kill Assay |
|||
1. INTRODUCTION
Oral health is crucial for general health and the quality of life of the individual. Vlachojannis et al. (2018) According to World Health Organization (WHO) 80 percentage of the world’s population presently uses herbal medicines for some aspect of primary health care. World Health Organization (2011) Natural products have been used since decades for various purposes. Today plants are being used in maintaining health, diagnosis, prevention and in the treatment of physical illness such as arthritis, kidney diseases, migraine, allergies, skin diseases, wounds, burns, gastrointestinal issues and even cancer as well as in mental illness. Paulraj et al. (2013)
Periodontitis is defined as an inflammatory disease of supporting tissues
of teeth caused by specific microorganisms resulting in progressive destruction
of the periodontal ligament and alveolar bone with periodontal pocket
formation, gingival recession, or both. Newman et al. (2006)
Periodontal pockets accommodate a number of
bacterial phylotypes including commensals and true pathogens. Porphyromonas gingivalis
(Pg) and Aggregatibactor actinomycetemcomitans
(Aa) have been detected in large numbers in periodontal pocket. Three
species, Pg, Aa and Bacteriodes forsythus were strongly associated with progressive
status of the periodontal disease and unsuccessful therapy. Socransky & Haffajee (2002) Scaling and
root planing (SRP) is considered as the gold standard
for the treatment of periodontitis. Newman et al. (2006)
However, treated sites are subjected to recolonization within few months
with a microbiota similar to that present before
therapy. Mombelli (2018) So, adjuvant
therapies such as antibiotic therapy are mainly used to combat such
microbes but due to their misuse, microbes develop increased resistance to many
common antibiotics. Vlachojannis et al. (2018)
Synthetic antiseptics are currently in use to reduce the bacterial load and
chlorhexidine (CHX) is considered as the gold standard in dentistry. Vlachojannis et al. (2018) However, there are drawbacks to the use of
CHX which includes staining of teeth and mucosa, dysgeusia, as well as long
term usage of CHX may lead to the emergence of new resistant staphylococci
strains. Vlachojannis et al. (2018) In light of the
growing antibiotic resistance, the usage of plant-derived antimicrobial agents
could serve as an effective alternative treatment against oral infections.
The genus Spilanthes belongs
to the family Asteraceae also known as family Compositae which is
comprised of more than 300 species. Paulraj et al. (2013)
Different species in this genus are found to have a
wide range of therapeutic and medicinal properties such as hepatoprotective and
diuretic properties. Paulraj et al. (2013)
Spilanthes acmella
(S. acmella) which is also known as
eyeball plant, spot plant and Para cress, is a vital medicinal plant
prominently distributed in the tropical and subtropical regions around the
world including India. Abdul Rahim et al. (2021)
These plants have been popularly called toothache plant, the reason being its
major use for toothache where the fresh flower head and/or leaves are chewed or
placed in the cavities of decayed teeth which relieves the pain with its
anesthetising property. Abdul Rahim et al. (2021)
All parts
of the plant are bitter in taste, with the flower heads being the most pungent
part which on consuming causes a tingling sensation, numbness, and excess
salivation. Abdul Rahim et al. (2021)
S. acmella is known to be a rich source of important bioactive compounds and these bioactive compounds have been used for its various properties such as anti- inflammatory, antibacterial, antifungal, antinociceptive, anti-cancer activities, and promote wound healing. Prachayasittikul et al. (2013) Spilanthol, which is N-isobutylamide, is the major phytochemical present in S. acmella which is responsible for its various biological activities. Yasuda et al. (1980) S. acmella offers active metabolites called phenolics, including as vanillic acid, trans-ferulic acid, trans-isoferulic acid, and stigmasteryl glucoside, which are highly effective antioxidants. Prachayasittikul et al. (2009) The bioactive substances from each part of S. acmella have been shown in the literature to have exceptional pharmacological activity. Prachayasittikul et al. (2009)
To the investigator’s knowledge, literature pertaining to the antimicrobial activity of S. acmella on periodontal pathogens has not been reported earlier. Therefore, the present study was conceptualized as an initial step to evaluate the minimal inhibitory concentration of S. acmella against common periodontal pathogens.
In this in vitro study, Spilanthes acmella extract was evaluated for,
· To assess the antibacterial activity of S. acmella plant extract against Porphyromonas gingivalis (Pg), and Aggregatibactor actinomycetemcomitans(Aa).
· To determine the presence of various phytochemicals.
2. MATERIALS AND METHODS
2.1. Preparation
of extract
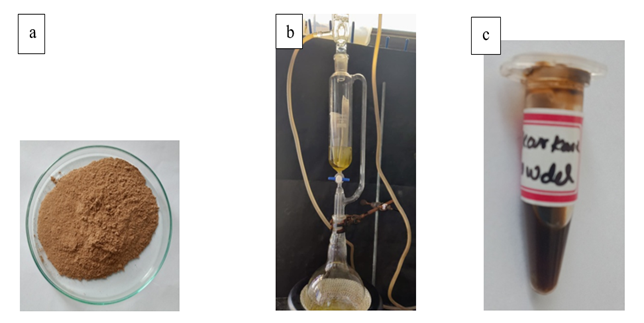
S. acmella plants were obtained from local dealer under aseptic conditions and the specimens were identified by a botanist for their authenticity. Flowerheads, leaves, stems and roots were seperated from the plant, washed in distilled water and dried in the sun for a week. They were then blended using electric blender to obtain a fine powder. Methanol extract of S. acmella was prepared in 1:1 (w/v) ratio using Soxhlet apparatus. Charu et al. (2022) 50g of powdered sample was filled into a thimble and subjected to Soxhlet extraction using 150ml of 99% methanol as solvent. The extract was concentrated using rotary evaporator and placed in incubator for 24 h at room temperature. After 24 hrs, the mixtures were filtered through 8 layered muslin cloth filter and centrifuged at 5000 rpm for 15 min. Charu et al. (2022) The supernatants were collected and the solvents were evaporated to make the final volume one-fourth of the original in a rotary evaporator at 4 rpm, 75 torr, and 50°C. Charu et al. (2022) Then the extracts were stored at 4̊ C in airtight bottles for further use. Charu et al. (2022) [Figure 1(a), 1(b), 1(c) and 1(d)]
Figure 1
|
Figure 1 Extraction of Spilanthes Acmella Plant: (a) Powder of the Whole Plant (b) Soxhlet Apparatus (c) Spilanthes Acmella Plant Extract |
2.2. Minimal Inhibitory Concentration
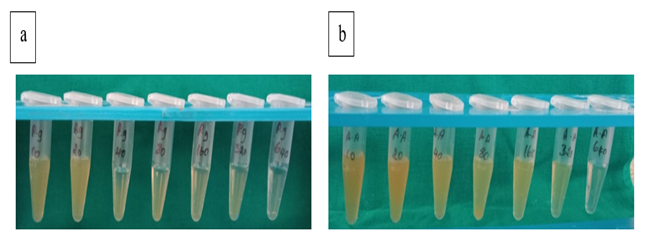
Minimum inhibitory concentration (MIC) of this extract was estimated using serial dilutions of the agent by tube dilution method. Respective strains of Pg and Aa were chosen for the study [Table 1]. The tubes were incubated for 24 h at 37°C. The optical density of each tube was evaluated after incubation using a spectrophotometer (Labman, India) at 600 nm at 37°C for 24 hours. Chaiya et al. (2013) The minimum concentration that repressed 100% growth of Pg and Aa was indicated as MIC. Briefly, concentrations from 10 μg/ml to 640 μg/ml of extract was added into the tubes containing 300 μL of thioglycollate broth following the method used by Chaiya et al. (2013) From the maintained stock cultures of Pg and Aa, 100 µL was taken and added into 2 ml of thioglycollate broth. In each serially diluted tube 100 μL of above culture suspension was added.[12] The tubes were incubated for 48–72 h in an anaerobic condition at 37°C and observed for turbidity. Chaiya et al. (2013) The respective samples were tested for Optical density at 600nm and tabulated. [Figure 2 (a) and (b)]
Table 1
|
Table 1 Strains Used in the Study |
||
|
S. No |
Test Organisms |
Strain |
|
1 |
Porphyromonas gingivalis |
ATCC33277 |
|
2 |
Aggregatibacter actinomycetemcomitans |
ATCC29522 |
Figure 2
|
Figure 2 Serial Dilutions of Spilanthes Acmella Extract (a) Porphyromonas Gingivalis, (b) Aggregatibacter Actinomycetemcomitans |
2.3. Time Kill
Assay
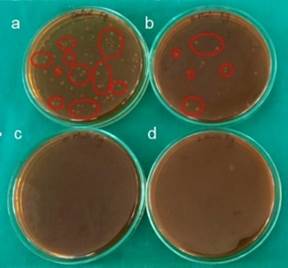
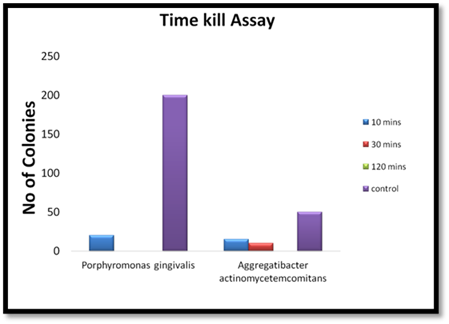
Following the MIC results, equal quantity of the broth with organisms (Pg and Aa) and plant extract was mixed which was then plated immediately and this was noted as 0 h [control tubes]. Appiah et al. (2017) Tubes were kept in anaerobic conditions till further time slot, that is, 10 min, 30 min, and 2 h. It was cultured and incubated according to the growth requirement. Appiah et al. (2017) After 48–72 h of incubation, the plates were removed and the number of colonies was noted Appiah et al. (2017) [Figure 3 and Figure 4].
Figure 3
|
Figure 3 Time Kill Assay- Colonies of P. Gingivalis (a) at Baseline (b) after 10 min (c) after 30 min (d) after 2 hrs |
Figure 4

|
Figure 4 Time Kill Assay- Colonies of A. Actinomycetemcomitans (a) at Baseline (b) after 10 min (c) after 30 min (d) after 2 hrs |
Figure 5
|
Figure 5 Time Kill Assay- Graph |
2.4. Phytochemical
analysis
TLC analysis of plant extract was done and checked for the presence of various phytochemicals [Table 2]. It was performed on 20 cm x 10 cm silica gel aluminium plate. Mian et al. (2019) Two micro liters of the extract was deposited in a glass twin-trough chamber previously saturated with mobile phase vapour for 20 min and hexane: ethyl acetate: formic acid in the ratio of 7:10:0.1 (v/v/v) was used as mobile phase. Mian et al. (2019) After development, the plates were dried with a hair dryer and then the spotted TLC plates visualized in the day light and under the UV wavelength (short & long) i.e. 254 nm, 366 nm respectively. Mian et al. (2019)
The Rf value (retardation factor) of spots was
determined by the given formulae. Mian et al. (2019)
Rf Value=Distance travelled by spot / Distance travelled by solvent.
Table 2
|
Table 2 Various Phytochemicals Tested in the Extract |
|
|
S. No |
Phytochemicals |
|
1 |
Alkaloid |
|
2 |
Flavonoid |
|
3 |
Glycoside |
|
4 |
Tannin |
|
5 |
Saponin |
|
6 |
Steroids |
|
7 |
Phenol |
|
8 |
Terpenoids |
3. RESULTS
In the present study, both the periodontal pathogens, namely, Pg and Aa were found sensitive to S. acmella methanolic extract. Aa was sensitive until 20 μg/ml dilution and showed resistance to further dilution. Pg was sensitive until 40 μg/ml dilution and showed resistance to further dilution. [Table 3 and Table 4] Time kill curve assay showed that Pg was inhibited within 30 min and Aa within 2 h [Table 5]. Various phytochemicals were found in the S. acmella extract i.e., Alkaloid, Flavonoid, Glycoside, Tannin, Saponin, Steroids, Phenol and Terpenoids. [Table 6].
Table 3
|
Table 3 MIC of Spilanthes Acmella Extract Against Pg |
||
|
Extract Concentration
(µg/ml) |
MIC(µg/ml) OD at 600 nm |
% Reduction (Pg) |
|
10 |
1.433 |
27.03 |
|
20 |
1.325 |
32.53 |
|
40 |
0.756 |
61.50 |
|
80 |
0.675 |
65.63 |
|
160 |
0.243 |
87.62 |
|
320 |
0.232 |
88.18 |
|
640 |
0.213 |
89.15 |
|
Control (Without Sample only
Pg) |
1.964 |
|
|
(MIC: minimal inhibitory concentration, Pg: Porphyromonas
gingivalis) |
||
Table 4
|
Table 4 MIC of Spilanthes Acmella Extract Against Aa |
||
|
Concentration (µg/ml) |
MIC (µg/ml) OD at 600 nm |
% Reduction (Aa) |
|
10 |
0.997 |
44.45 |
|
20 |
0.806 |
55.09 |
|
40 |
0.456 |
74.59 |
|
80 |
0.302 |
83.17 |
|
160 |
0.156 |
91.31 |
|
320 |
0.155 |
91.36 |
|
640 |
0.145 |
91.92 |
|
Control (Without Sample only
Aa) |
1.795 |
|
|
(MIC: minimal inhibitory concentration, Aa: Aggregatibactor
actinomycetemcomitans) |
||
Table 5
|
Table 5 Time Kill Assay |
||||
|
Time
Kill Assay |
||||
|
Organism |
No.
of colonies seen after |
|||
|
10
min |
30
min |
120
min |
No.
of colonies in positive Control |
|
|
Porphyromonas gingivalis |
20 |
0 |
0 |
2×102 |
|
Aggregatibacter actinomycetemcomitans |
15 |
10 |
0 |
0.5×102 |
Table 6
|
Table 6 Various Phytochemicals in the Extract with their Percentage |
||
|
S. No |
Phytochemicals |
Methanol
Extract (%) |
|
1 |
Alkaloid |
0.76 |
|
2 |
Flavonoid |
0.7 |
|
3 |
Glycoside |
0.84 |
|
4 |
Tannin |
0.6 |
|
5 |
Saponin |
0.98 |
|
6 |
Steroids |
0.7 |
|
7 |
Phenol |
0.73 |
|
8 |
Terpenoids |
0.62 |
4. DISCUSSION
As far as the investigator is aware, there hasn't been any prior reporting of literature on S. acmella's antimicrobial efficacy against periodontal pathogens. Since no direct tests have been conducted, the current study's goal was to ascertain the antimicrobial efficacy of the extract against Pg and Aa using tube dilution method and time kill curve and the presence of various phytochemicals in the S. acmella whole plant extract. Our data confirm that S. acmella is a potent inhibitor of Pg as well as Aa. In addition, we found that at a minimum concentration of 40 μg/ml, both the periodontal pathogens were inhibited. The extract from S. acmella was shown to have a minimum inhibitory concentration of 40 μg/ml for Pg and 20 μg/ml for Aa. Both of the studied organisms did not develop within two hours, according to the time kill curve experiment.
A study done by Shobana G on Anti-bacterial efficacy of S. acmella on salivary mutans Streptococci in which it was found that 20% Methanolic extract of S. acmella was as efficacious as chlorhexidine as an antimicrobial agent on salivary mutans Streptococci. Shobana (2018) When compared to medications like Ca (OH)2, Sathyaprasad et al. (2015)'s study revealed that S. acmella possesses remarkable antibacterial and antifungal activity against common root canal pathogens, such as Enterococcus faecalis and Candida albicans, which are responsible for recurrent endodontic failures. Sathyaprasad et al. (2015) The examined bacteria and fungus were significantly inhibited by the crude extracts of S. acmella. In one investigation by Ahmed S et al., the antimicrobial activities were modest against Salmonella typhi, Stapylococcus aureus, and Bacillus subtili, but they had strong antifugal activity against three fungi, namely Candida albicans, Aspergillus niger, and Sacharomyces cerevacae. Ahmed et al. (2012)
A study done by Praveen NC et.al. Aa and Pg were found to be sensitive to pineapple
extract (bromelain) at a minimum concentration of 16.6 mg/ml and 4.15 mg/ml,
respectively. Praveen et al. (2014) Another study conducted by Patra JK
et.al., the crude extract and the two fractions (chloroform and hexane)
of Robinia pseudoacacia were
proved highly active in controlling Pg.
Patra et al. (2015) According to Müller-Heupt
et al. (2022), an ethanolic Azadirachta indica leaf extract had a MIC of 1024
mg/L and 256 mg/L for the acetone extract against Pg ATCC 33277. A 100%
ethanolic extract of Rheum palmatum root
showed a MIC of 4 mg/L for Pg ATCC
33277. The MIC of Eucalyptus globulus leaf extracts in acetone and ethanolic
were determined to be 128 mg/L and 256 mg/L, respectively, against Pg. [20]
Verma K et. al have done a similar study where they have used Acmella oleracea (similar species to S. acmella) in gel form clinically as a local drug
delivery (LDD) and have found significant improvement in clinical parameters
when combined with SRP. Verma et al. (2022)
A study done by Ramsevak et.al.
showed that hexane extract of flower buds of S. acmella contained
three N-isobutyl
amides: spilanthol, undeca-2E,7Z,9E-trienoic
acid isobutylamide and undeca-2E-en-8,10-diynoic acid isobutylamide. Ramsewak et al. (1999) Qualitative
phytochemical screening of S. acmella extracts
done by Rao TM et.al. demonstrated the existence of many phytochemical
components, such as steroids, terpenoids, flavanoids,
alkaloids, glycosides, tannins, carbohydrates, oils, and amino acids. Also, the
methanolic extract had more phenolic content when compared to other extracts. Rao et al. (2012)
Nakatani and Nagashiwa demonstrated
that the existence of amides- spilanthol and alkamides that may be the cause of the antibacterial and
antifungal action of various concentrations of S. acmella
extract. Nakatani & Nagashiwa (1992)
presence of
nonvolatile sesquiterpenoids and saponins were also
reported by Krishnaswami et al. and Mukharya et al.
as potentially contributing to the antibacterial and antifungal properties of S.
acmella. Krishnaswami et al. (1975), Mukharya & Ansari (1986)
5. CONCLUSION
The S. acmella extract shows a significant antibacterial effect against the major periodontal pathogens i.e. Pg and Aa and hence may be a potential natural alternative for controlling the growth of these bacteria. S. acmella possess various phytochemicals which could be responsible for its anti- bacterial property. In conclusion, the genus Spilanthes offers a wide range of research possibilities. To ascertain its therapeutic effectiveness and suitability for incorporation into regular at-home oral hygiene products, more in vivo research studies are required.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
Abdul Rahim, R., Jayusman, P.A., Muhammad, N., Mohamed, N., Lim, V., Ahmad, N.H., Mohamad, S., Abdul Hamid, Z.A., Ahmad, F., Mokhtar, N., &Shuid, A.N. (2021, Mar 29). Potential Antioxidant and Anti-Inflammatory Effects of Spilanthes Acmella and Its Health Beneficial Effects: A Review. International Journal of Environmental Research and Public Health, 18(7), 3532. https://doi.org/10.3390/ijerph18073532
Ahmed, S., Rahman, A., Muslim, T., Sohrab, M.H., Akbor, M.A., Siraj, S., Sultana, N., & Al-Mansur, M.A. (2012). Antimicrobial Cytotoxicity and Phytochemical Activities of Spilanthes Acmella. Bangladesh Journal of Scientific and Industrial Research, 47(4),437-40. https://doi.org/10.3329/bjsir.v47i4.14073
Appiah, T., Boakye, Y. D., & Agyare, C. (2017). Antimicrobial Activities and Time-Kill Kinetics of Extracts of Selected Ghanaian Mushrooms. Evidence-Based Complementary and Alternative Medicine, 15. https://doi.org/10.1155/2017/4534350
Chaiya, A., Saraya, S., Chuakul, W., & Temsiririrkkul, R. (2013). Screening for Dental Caries: Preventive Activities of Medicinal Plants against Streptococcus Mutans. Mahidol University Journal of Pharmaceutical Sciences, 40(1), 9-17.
Charu, F., Tannukit, S., Rotpenpian, N., & Jitpukdeebodintra, S. (2022, May 1). Cytotoxicity and Cell Migration Effect of Crude Spilanthes Acmella Ethanolic and Water Extract. Journal of International Dental and Medical Research, 15(2, 544-51.
Krishnaswami, N.R., Prasanna, S., Seahadri, T.R., & Vedantham, T.N.C. (1975). α and β- Amyrin Esters and Sitosterol Glucoside from Spilanthes Acmella Phytochemistry, 14, 1666-7. https://doi.org/10.1016/0031-9422(75)85386-6
Mian, S.S., Upadhayay, S., & Tajuddin, S.N. (2019, Jan 1). Physicochemical Analysis of Ginger (Zingiber officinale Rosc.) Rhizome Along with its TLC, HPLC and HPTLC Profile. Pharmaceutical Methods, 10(1). https://doi.org/10.5530/phm.2019.1.6
Mombelli, A. (2018). Microbial Colonization of the Periodontal Pocket and Its Significance for Periodontal Therapy. Periodontology 2000, 76, 85-96. https://doi.org/10.1111/prd.12147
Mukharya, D.K.K., & Ansari, A.H. (1986). Olean-12-en-3-O-ß D-Galactopyranosyl (1-4)-O-a-l- rhamnopyranoside: A New Triteropenoidal Saponin from the Roots of Spilanthes Acmella (Murr). Indian J Chem, 26, 81-7.
Müller-Heupt, L.K., Vierengel, N., Groß, J., Opatz, T., Deschner, J., & Von Loewenich, F.D. (2022, Jan 31). Antimicrobial Activity of Eucalyptus Globulus, Azadirachta Indica, Glycyrrhiza Glabra, Rheum Palmatum Extracts and Rhein Against Porphyromonas Gingivalis. Antibiotics, 11(2), 186. https://doi.org/10.3390/antibiotics11020186
Nakatani, N., & Nagashiwa, M. (1992). Pungent Alkamides from Spilanthes Acmella Biosci Biotechnol Biochem, 56, 759-62. https://doi.org/10.1271/bbb.56.759
Newman, M.G., Takei, H., Kokkevold, P.R., & Carranza, F.A. (2006). Clinical Periodontology 10th edition. St. Louis: Saunders-Elsevier, 139-141. https://www.amazon.in/Carranzas-Clinical-Periodontology-dition-Continually/dp/1416023992
Patra, J.K., Kim, E.S., Oh, K., Kim, H.J., Dhakal, R., Kim, Y., & Baek, K.H. (2015, Apr 8). Bactericidal Effect of Extracts and Metabolites of Robinia Pseudoacacia L. on Streptococcus Mutans and Porphyromonas Gingivalis Causing Dental Plaque and Periodontal Inflammatory Diseases. Molecules, 20(4), 6128-39. https://doi.org/10.3390/molecules20046128
Paulraj, J., Govindarajan, R., & Palpu, P. (2013 Dec 26). The Genus Spilanthes Ethnopharmacology, Phytochemistry, and Pharmacological Properties: A Review. Advances in Pharmacological Sciences. https://doi.org/10.1155/2013/510298
Prachayasittikul, S., Suphapong, S., Worachartcheewan, A., Lawung, R., Ruchirawat, S., Prachayasittikul, V. (2009, Feb 19). Bioactive Metabolites from Spilanthes Acmella Murr. Molecules, 14(2), 850-67. https://doi.org/10.3390/molecules14020850
Prachayasittikul, V., Prachayasittikul, S., Ruchirawat, S., & Prachayasittikul, V. (2013). High Therapeutic Potential of Spilanthes Acmella: A Review. EXCLI Journal,12, 291-312. https://pubmed.ncbi.nlm.nih.gov/27092032/
Praveen, N.C., Rajesh, A., Madan, M., Chaurasia, V.R., Hiremath, N.V., & Sharma, A.M. (2014 Sep). In Vitro Evaluation of Antibacterial Efficacy of Pineapple Extract (Bromelain) on Periodontal Pathogens. Journal of International Oral Health: JIOH, 6(5), 96-98.
Ramsewak, R.S., Erickson, A.J., & Nair, M.G. (1999). Bioactive N-Isobutylamides from the Flower Buds of Spilanthes Acmella. Phytochemistry, 51, 729-732. https://doi.org/10.1016/S0031-9422(99)00101-6
Rao, T.M., Rao, B.G., & Rao, Y.V. (2012). Antioxidant Activity of Spilanthes Acmella Extracts. Int J Phytopharm, 3(2), 216-0. https://doi.org/10.1016/S2222-1808(12)60153-4
Sathyaprasad, S., Jose, B.K., & Chandra, H.S. (2015, Sep 1). Antimicrobial and Antifungal Efficacy of Spilanthes Acmella as an Intracanal Medicament in Comparison to Calcium Hydroxide: An in Vitro Study. Indian Journal of Dental Research, 26(5), 528. https://doi.org/10.4103/0970-9290.172081
Shobana, G. (2018). Anti-Bacterial Efficacy of Spilanthes Acmella on Salivary Mutans Streptococci in 15-17 Years Old School Students in Madurai District: A Randomized Controlled Trial (Doctoral Dissertation, Best Dental Science College, Madurai).
Socransky, S.S., & Haffajee, A.D. (2002, Jan). Dental Biofilms: Difficult Therapeutic Targets. Periodontology 2000, 28(1), 12-55. https://doi.org/10.1034/j.1600-0757.2002.280102.x
Verma, K., Dhruvakumar, D., & Pande, M. (2022, May). A Clinical and Microbiological Study to Assess the Efficacy of Acmella Oleracea and Acacia Catechu Herbs as Local Drug Delivery in Treatment of Chronic Generalized Periodontitis Patients. Journal of Indian Society of Periodontology, 26(3), 254. https://doi.org/10.4103/jisp.jisp_264_21
Vlachojannis, C., Chrubasik-Hausmann, S., Hellwig, E., Vach, K., & Al-Ahmad, A. (2018). Activity of Preparations from Spilanthes Oleracea, Propolis, Nigella Sativa, and Black Garlic on Different Microorganisms Involved in Oral Diseases and on Total Human Salivary Bacteria: A Pilot Study. Phytotherapy Research, 32(10), 1992-2001. https://doi.org/10.1002/ptr.6129
World Health Organization (2011). Quality Control Methods for Herbal Materials. World Health Organization.
Yasuda, I., Takeya, K., & Itokawa, H. (1980, Jul 25). The Geometric Structure of Spilanthol. Chemical and Pharmaceutical Bulletin, 28(7), 2251-3. https://doi.org/10.1248/cpb.28.2251
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© Granthaalayah 2014-2024. All Rights Reserved.