|
|
|
|
GREEN SYNTHESIS, ANTIBACTERIAL SCREENING AND ANTIOXIDANT ACTIVITY OF SILVER NANOPARTICLES (AgNPs) CAPPED WITH METABOLITES FROM Andrographis paniculata (Burm.f.) Wall. ex Nees LEAVES
Welven Segumpan 1![]()
![]() ,
Remedel Dela Mines 2
,
Remedel Dela Mines 2![]()
![]() ,
Aprille Mae Bunuan 1
,
Aprille Mae Bunuan 1![]()
![]() ,
Maria Theresa Marlyn Ballesteros 1
,
Maria Theresa Marlyn Ballesteros 1![]() , Elsa Cajucom 3
, Elsa Cajucom 3![]()
![]()
1 Student,
School of Graduate Studies, Saint Mary's University, Bayombong, 3700 Nueva
Vizcaya, Teacher, Prathomsuksa, Philippines, Thammasat
School, Thammasat University, Rangsit, Pathumthani, Thailand
2 Student,
School of Graduate Studies, Saint Mary's University, Bayombong, 3700 Nueva
Vizcaya, Philippines
3 Director, Center for Natural Sciences, Saint Mary's University,
Bayombong, 3700 Nueva Vizcaya, Philippines
|
|
ABSTRACT |
||
|
The study
presents a novel method for synthesizing silver nanoparticles (AgNPs) using A. paniculata leaves extract as a bioreducing agent for Ag+ ions derived from AgNO3. The
biomolecules within the extract are credited with the reduction process.
Characterization techniques including UV-Vis
spectroscopy, Fourier Transform Infrared (FTIR) spectroscopy, X-ray
Diffraction (XRD) analysis, and Scanning Electron Microscopy-Energy
Dispersive X-ray (SEM-EDX) analysis were employed to analyze the properties
of the synthesized nanoparticles. UV-Vis
spectroscopy revealed a prominent Surface Plasmon Resonance (SPR) peak at 550
nm, indicative of the presence of AgNPs with
efficient light absorption and scattering properties. SEM analysis provided
insights into the morphology and size distribution of the nanoparticles. XRD
analysis confirmed the crystalline nature of the nanoparticles, while EDX
analysis corroborated the presence of elemental silver in the nanoparticle
composition. The antimicrobial activity of the synthesized AgNPs against a spectrum of human pathogens, particularly
noteworthy inhibition against E. coli and S. aureus, highlights their
potential as antimicrobial agents. Furthermore, the antioxidant activity
assessed through the DPPH scavenging assay underscores the potential health
benefits of these nanoparticles. A notable observation was the variation in
activity between A. paniculata extract and A. paniculata-AgNPs,
with the latter exhibiting reduced inhibitory effects attributed to fewer
functional groups on the nanoparticle surface. This finding contributes to a
deeper understanding of structure-function relationships in
nanoparticle-based applications. |
|||
|
Received 25 March 2024 Accepted 28 April 2024 Published 17 May 2024 Corresponding Author Welven Segumpan, hed-wsegumpan@smu.edu.ph
DOI 10.29121/ijetmr.v11.i5.2024.1427 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2024 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Green
Synthesis, Nanotechnology, Silver Nanoparticles, Metabolites |
|||
1. INTRODUCTION
The field
of nanotechnology is the most active area of research in modern material
sciences. Though there are many physical and chemical methods,
environment-friendly synthesis is the most emerging method of synthesis. One of
the environment-friendly methods is green synthesis that has many possible
applications in environmental and biomedical fields. Green synthesis is
considered as a key tool in reducing the destructive effects associated with
the traditional methods of synthesis for nanoparticles commonly utilized in
laboratory and industry Singh et al. (2018).
Plant-based extracts have drawn consideration over conventional due to
its numerous advantages such as non-hazardous, economical, and feasible methods
with variety of applications in biomedicine, and nanotechnology, etc.
Nanoparticles
have unique physicochemical properties such as high surface area, high
reactivity, tunable pore size, and particle morphology. Nanoparticles can serve as “magic bullets”,
containing herbicides, nano-pesticide fertilizers, or genes, which target
specific cellular organelles in plant to release their content. So,
nanoparticle synthesis from plant extracts tentatively offers a route for large
scale production of commercially attractive nanoparticles. Silver nanoparticles
(AgNPs), among other nanoparticles, acquired more
attention because of their unique properties Alharbi et al. (2022).
Silver particles are used in different fields including medical, food,
healthcare, consumer and industrial, as antibacterial agents, medical device
coatings, optical sensors, anticancer agents, etc. Its applications in the life
sciences and medicine have focused on antibacterial, antifungal, antiviral,
anti-inflammatory, anticancer, and antiangiogenic properties. Thus, AgNPs are reported to have potential anticancer,
antimicrobial, antioxidant, antifungal, anti-inflammatory, antiviral,
antiangiogenetic, and antiplatelet activities Luhata et al. (2022). Different studies of plant
extracts used for the synthesis of AgNPs were in the literature namely Memecylon
edule, Callicarpa maingayi,
Terminalia chebula, Trachyspermum ammi
and Papaver somniferum, Bauhinia variegate L., Hevea brasiliensis, Aloe vera and tea leaf Keshari et al. (2020). Andrographis paniculata (Burm.f.) Wall. ex
Nees in Wall belong to the family Acanthaceae. This plant used as traditional medicine to
treat infectious diseases and fevers. Andrographis paniculata grows in
moist and shady places. This plant is erect with 30 -110 cm in height. It has
slender stem, dark - green lance - shaped leaves. The blades measure from 8 cm
long by 2.5 cm wide. The small flowers produce a capsule from 2cm long and few
millimeters wide. Yellow brown seeds ae within these capsules Kumar et al. (2021). This paper will use extracts from Andrographis paniculata for the AgNPs that may be a possible source of antimicrobial or
antioxidant, anti – inflammatory or antifungal properties.
The use
of Andrographis paniculata as an effective medicine lasted for
centuries especially in Asia. Diseases related to blood abnormalities like skin
eruptions, boils, scabies and chronic undetermined
fever are treated because of its “blood purifying” effect. The presence of
chemical like lactones, diterpenoids, diterpene, glycosides, flavonoids and
flavonoid glycosides made this plant a common treatment for upper respiratory
tract infection.
The
utilization of Andrographis paniculata
as a medicinal plant for treating a number of diseases
in most Asian countries poses critical evaluation since these cases are
considered self- limiting Okhuarobo et al. (2014). In the study conducted by Jayakumar et al. (2013), the leaves contained the highest
amount of andrographolide, the bitter constituent of the leaves. In addition,
this chemical is present in great amount in leaves Jayakumar et al. (2013). The range is from12.44 to 33.52
mg/g in dried leaves found at 90 - 120 days. It is on this premise that the
leaves are used for the characterization and antioxidant activity of silver
nanoparticles (AgNPs) capped with metabolites from Andrographis paniculata leaves.
2. MATERIALS AND METHODS
2.1. Materials and Tools
Plant
samples was collected from Purok 3, Ibung,
Villaverde, Nueva Vizcaya. The plant specimen was identified and authenticated
at the Institute of Biology, Jose Vera Santos Memorial Herbarium (PUH), College
of Science, University of the Philippines, Diliman, Quezon City. Aqueous
solution of silver nitrate (AgNO3, 1mM) was freshly prepared at SMU CNS
Laboratory from Merck.
The A.
paniculata-AgNPs was characterized using BK
UV 1000 Spectrophotometer,
Nicolet 6700 FT-IR Spectrometer, PHYWE X-ray diffraction equipment and JEOL
5310 scanning electron microscope (SEM) - AMETEK EDAX ELEMENT Energy
Dispersive Spectroscopy (EDS) System.
2.2. Method of Qualitative and Quantitative Analysis
The study
utilized the quantitative research using the experimental method used to gather
data and to guarantee the accuracy and reliability of the data vital to attain
substantial and thorough interpretations of the results.
The
research focused on the development of a method for the synthesis of AgNPs that do not include the use of toxic and flammable
chemicals. This study made use
of Andrographis paniculata, also known as Serpentina. The
experiment was conducted at Saint Mary’s University Center for Natural Sciences
Laboratory in December 2022.
Procedures
Extract Preparation
The plant samples were washed with distilled water
and oven dried. After drying, the leaves then crushed and soaked in distilled
water for 24 hr. at room temperature. The extract was filtered using Whatman
filter paper to separate the solid powder affording a clear light-yellow
solution.
Biosynthesis of Silver Nanoparticles
Biosynthesis of AgNPs was
performed following the procedure used by Song & Kim (2008). A freshly prepared
aqueous solution of silver nitrate (AgNO3, 1mM) was used for AgNPs synthesis. Plant extracts (5mL) will be mixed with a
45 mL AgNO3 solution. To observe the effect of temperature on the synthesis of AgNPs, biosynthesis will be carried out at room temperature
and 60C.
Characterization of AgNPs
UV-Vis Spectroscopic Analysis
UV-Vis spectroscopic analysis of biosynthesized AgNPs has been performed by continuous scanning from 250 to
750 nm and 1 mM AgNO3 solution was used for baseline correction.
X-Ray Diffraction (XRD) analysis
X-ray diffraction (XRD) analysis of purified AgNPs was performed to describe the diffraction pattern pf
the biosynthesized AgNPs.
Fourier Transform Infrared (FTIR) analysis
The fine powder of the biosynthesized AgNPs was analyzed using FTIR to study the biomolecules
presence as capping agents on AgNP’s surface.
Scanning Electron Microscopy (SEM) and Electron
Diffraction Spectroscopy (EDX)
SEM-EDX analysis of purified AgNPs
was performed to describe the morphology of the biosynthesized AgNPs.
Free Radical Scavenging Activity
Samples (1 mL) contains different concentrations
(50, 100, 150, 200, 250, 300, 350, and 400 uL/mL) of AgNPs, were mixed with 1 mL of freshly prepared DPPH
(0.004% w/v in absolute methanol) solution. The reaction solution was incubated
for 30 minutes in the dark at room temperature. Absorbance has been recorded at
517 nm using UV-Vis spectrophotometer. Methanol was
used as blank, and DPPH was used as control. Free radical scavenging activity
was expressed as the percentage of inhibition.
Antibacterial screening
E. coli, and S. aureus was used for the
antibacterial screening of AgNPs.
3. RESULTS AND DISCUSSIONS
3.1. Result of Qualitative and Quantitative Analysis
1)
UV-Vis
Spectroscopic analysis
UV-Vis Spectrophotometer was used to detect the
visual characteristic and spectrum absorption of the biosynthesized silver
nanoparticles. Table 1 shows the absorbance
values of the silver nanoparticles.
Table 1
|
Table 1 The Average
Absorbance of the Raw Extract and Synthesized A. Paniculata-AgNPs |
|||
|
Wavelength,
nm |
Mean
absorbance, |
A. paniculata-AgNPs at room temp |
A. paniculata-AgNPs at 60oC |
|
250 |
3.000 |
3.000 |
3.000 |
|
350 |
3.000 |
3.000 |
3.000 |
|
450 |
3.000 |
3.000 |
3.000 |
|
550 |
3.000 |
3.696 |
2.060 |
|
650 |
3.000 |
3.000 |
2.398 |
|
750 |
3.000 |
2.081 |
1.377 |
After addition of A. paniculata extract to silver nitrate solution, a UV-Vis scan was
taken from 250 to 750 nm. The fixed ration of extract to metal ion solution led
the change of color due to the formation of silver nanoparticles. The change in color is primarily attributed to the Surface Plasmon
Resonance (SPR) phenomenon exhibited by the silver nanoparticles. SPR is a
collective oscillation of free electrons on the surface of metal nanoparticles
when they interact with electromagnetic radiation, such as visible light. This
interaction leads to the absorption of specific wavelengths of light and the
generation of a characteristic absorption spectrum.
Figure 1 shows the SPR peak
intensity determined spectrophotometrically using BK UV 1000 Spectrophotometer
at 550 nm. AgNPs absorb and scatter light with
extraordinary efficiency centered at 550 nm. The strong interaction with light
at 550 nm occurs because the conduction electrons on the silver nanomaterial
surface undergo a collective oscillation when they are excited by light at this
wavelength. Observation of this strong broad plasmon peak has been well
documented for various metal NPs, with sizes ranging all the way from 2 to 100
nm Henglein (1993). A different set of
conditions was performed at 60oC and the SPR was measured at room
temperature.
Figure 1
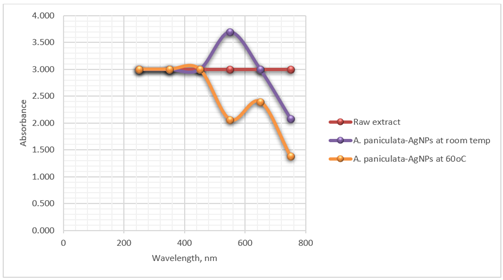
|
Figure
1 Optimal Synthesis Condition of
A. Paniculata-AgNPs |
2)
FTIR
Spectroscopic analysis
The infrared (IR) spectrum of A. paniculata exhibits
strong peaks at 1064.41 cm⁻¹ and 1136.32 cm⁻¹, indicating O-H
bending and C-O stretching vibrations, respectively, indicative of hydroxyl
(OH) and carbonyl (C=O) or ether (C-O-C) functional groups in the extract. A
peak at 3487.15 cm⁻¹ corresponds to O-H stretching and N-H extension
vibrations, suggestive of hydroxyl and amine (NH₂) or amide (NH-C=O)
groups, with mention of inter hydrogen bonds implying molecular hydrogen
bonding networks. These spectral features provide insights into the chemical
composition and structural characteristics of A. paniculata, aiding in
the identification of potential bioactive constituents and understanding its
pharmacological properties.
Figure 2
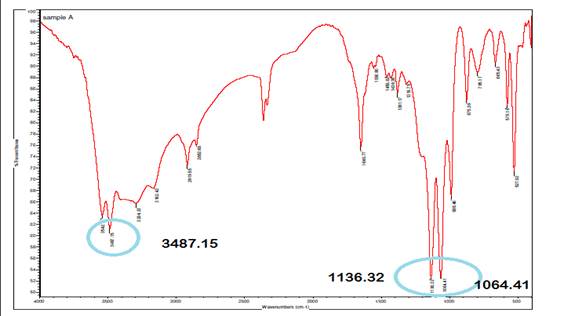
|
Figure 2 Infrared Spectrum of A. Paniculata Extract. |
In the infrared (IR) spectrum of A. paniculata
with Ag at 0H, the multiple bond region from 1500 cm⁻¹ to 2500 cm⁻¹
reveals numerous absorptions, particularly between 1500 cm⁻¹ to 2000
cm⁻¹, indicative of C-C aromatic bonds within the aromatic rings present
in the plant's organic compounds. Aromatic C-H stretching vibrations typically
appear at higher wavenumbers, specifically around 3100-3000 cm⁻¹, further
confirming the presence of aromatic hydrocarbons in the extract. These spectral
features are characteristic of compounds containing benzene rings or other
aromatic structures, providing valuable information about the aromatic
constituents in A. paniculata and contributing to the understanding of
its chemical composition and potential pharmacological activities.
Figure 3
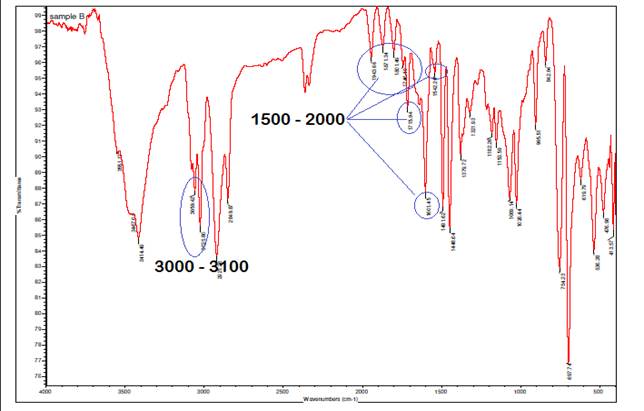
|
Figure 3 Infrared Spectrum of Interaction
of A. Paniculata with Ag at 0H. |
The presence of characteristic bands at 3000-3100 cm⁻¹,
corresponding to C-H stretching vibrations in aromatic compounds, along with
bands specific to phenyl groups at 1561.23 cm⁻¹, 1338.68 cm⁻¹, and
926.75 cm⁻¹ in the IR spectrum, serves as strong evidence confirming the
grafting of silver (Ag) onto the backbone of A. paniculata. The C-H
stretching bands in the 3000-3100 cm⁻¹ range are indicative of aromatic
hydrocarbons, aligning with the phenyl groups' vibrations at 1561.23 cm⁻¹
(C=C stretching), 1338.68 cm⁻¹ (C-H bending), and 926.75 cm⁻¹ (C-H
out-of-plane bending). These specific bands corroborate the integration of
silver nanoparticles onto the aromatic structure of A. paniculata,
providing insight into the interaction and bonding between Ag and the plant
extract, which is crucial for applications such as nanoparticle synthesis and
functionalization for various biomedical or environmental uses.
Figure 4
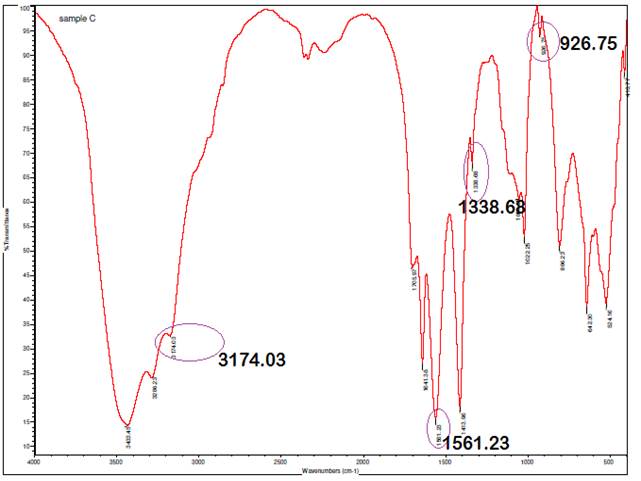
|
Figure 4 Infrared Spectrum of A.
Paniculata AgNPs at 1H |
Figure 5
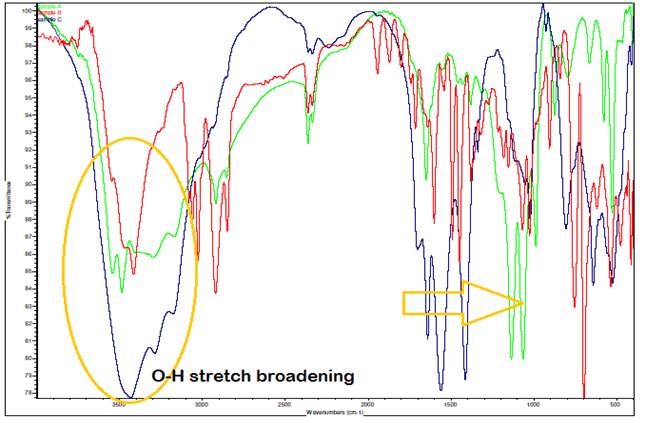
*green – A. paniculata extract, red – A. paniculata extract with Ag, blue – A. paniculata AgNPs
|
Figure 5 Comparison of IR Spectrum (Overlaid IR Spectra) |
The observed shift to slightly higher wavenumbers (rightward
shift) in the bands around 1500 cm⁻¹ in the IR spectrum, particularly in
the A. paniculata-AgNPs sample, can be
attributed to the phenomenon where heavier atoms tend to absorb at lower
frequencies. This shift indicates changes in molecular environments or
interactions involving heavier elements, possibly related to the presence of
silver nanoparticles (AgNPs) in the A. paniculata
extract. Additionally, the broadening of the O-H stretch compared to the
spectra of A. paniculata extract and A. paniculata extract with
Ag alone is attributed to the complex hydrogen bonding network within the A.
paniculata-AgNPs sample. Since a significant
quantity of compounds is present in the sample, each molecule may engage in
hydrogen bonding to varying extents, leading to a range of bond strengths and resulting in adsorptions at varying
frequencies during IR spectroscopy. This variation in bonding strengths and
frequencies contributes to the broadened appearance of the O-H stretch peak in
the IR spectrum, reflecting the average of these slightly different absorptions
across the sample.
3)
XRD Analysis
Figure 6
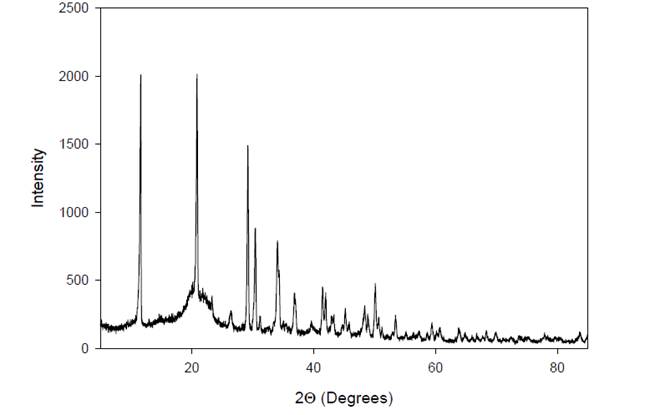
|
Figure 6 X-Ray Diffractogram of A. Paniculata Extract. |
The X-ray diffraction (XRD) profile of A. paniculata
extract reveals characteristic peaks at specific angles, represented as 2Θ
(2 times the angle of diffraction). In this case, the XRD pattern exhibits
peaks at 2Θ = 11º and 21º, indicating the crystallographic form of the
extract. These peaks correspond to the diffraction of X-rays by the crystalline
components present in the extract, providing information about their
arrangement and structure within the sample. The positions and intensities of
these peaks in the XRD pattern are unique to the crystalline phases present in
the extract, allowing for identification and characterization of the extract's
crystalline form.
Figure 7
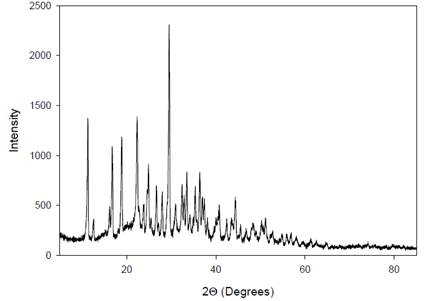
|
Figure 7 X-Ray
Diffractogram of the AgNPs. |
The X-ray diffraction (XRD) spectrum of the silver
nanoparticles (AgNPs) derived from A. paniculata
extract exhibits multiple crystalline areas between 11º-28º and 32º-45º in the
XRD pattern. This indicates that the AgNPs possess a
diverse range of crystal structures within these angular regions. The
complexity of crystal structures in the A. paniculata extract is
reflected in the XRD pattern, where each crystalline form exhibits its
distinctive peaks. These peaks are characteristic of the specific arrangement
of atoms in the crystal lattice, providing insights into the crystallographic
properties and structural diversity of the AgNPs
synthesized from A. paniculata extract.
Figure 8
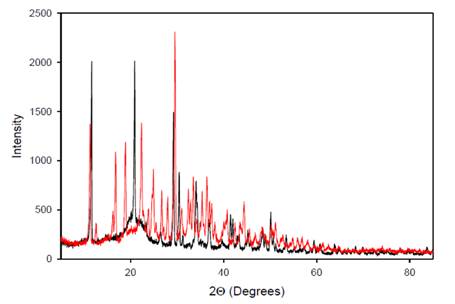
|
Figure
8 Overlaid X-Ray Diffraction
Patterns |
The overlaid peak intensities of A. paniculata extract
(black) and A. paniculata-AgNPs (red) in Figure 8 reveal an increase in the number of
peak intensities in the resulting diffractogram. This observation suggests that
the interaction of silver (Ag) with A. paniculata extract leads to the
formation of bonds contributing to several diffractions observed. The Bragg
reflections in the diffractogram indicate the presence of sets of lattice
planes, which can be indexed as a face-centered cubic
(FCC) structure typical of silver. This indexing is based on the arrangement of
atoms in the crystal lattice, confirming the crystalline nature of the silver
nanoparticles (AgNPs). The XRD differences between A.
paniculata-AgNPs and the A. paniculata
extract demonstrate that the crystallographic structure has changed
post-reduction, further supporting the formation of AgNPs.
Overall, the X-ray diffraction pattern clearly indicates that the AgNPs derived from A. paniculata extract are
crystalline in nature Shameli et al. (2011).
4)
SEM-EDX
Analysis
Figure 9
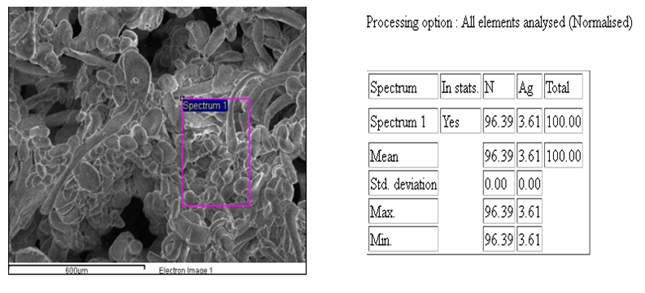
|
Figure 9 EDX Image of
AgNPs: Confirmatory Test for Ag |
The Energy Dispersive X-ray Spectroscopy (EDX) spectra of the
sample revealed that silver (Ag) and nitrogen (N) were the most abundant
elements present. This finding aligns with the synthesis process involving A.
paniculata extract and silver nitrate solution, where AgNPs
are formed with the assistance of nitrogen-containing compounds from the plant
extract. The EDX analysis provides quantitative information about the elemental
composition of the sample, highlighting the presence of Ag and N as the
predominant elements, which is consistent with the expected composition of A.
paniculata-AgNPs based on the synthesis method
used.
Figure 10
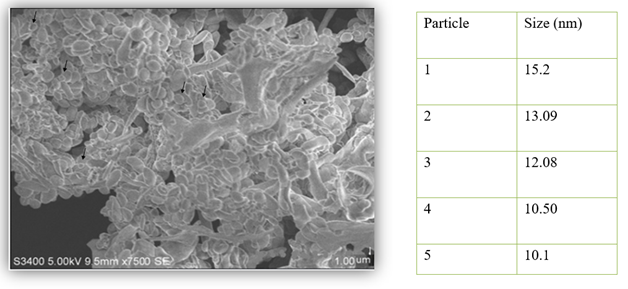
|
Figure 10 SEM
Micrograph of the AgNPs |
The Scanning Electron Microscopy (SEM) analysis of the sample provided insights into the surface morphology and actual size of the silver nanoparticles (AgNPs) synthesized using A. paniculata extract. The SEM images showed that the particles were polydispersed, indicating a range of sizes, and exhibited circular shapes. The high density of AgNPs observed in the SEM images, attributed to the A. paniculata extract, further confirmed the successful development of Ag nanostructures. This morphology and distribution of AgNPs are crucial for understanding their potential applications, as polydispersed nanoparticles with uniform shapes can offer enhanced properties in various fields such as catalysis, sensing, and biomedical applications.
5)
Antibacterial Assay
Table 2
|
Table 2
Antibacterial Assay of A. Paniculata-AgNPs |
||
|
|
Mean zone of inhibition |
|
|
|
Test organism |
|
|
|
Escherichia coli |
Staphylococcus aureus |
|
A. paniculata raw extract |
7.18 mm |
7.29 mm |
|
A. paniculata-AgNPs at room
temperature |
12.15 mm |
14.38 mm |
|
A. paniculata-AgNPs at 60oC |
11.06 mm |
14.18 mm |
|
Meropenem (positive control) |
28.56 mm |
23.19 mm |
|
Negative control |
6 mm |
6 mm |
|
Legend: <10 mm=inactive 10-13mm= partially active 14-19mm=active >19mm= very active |
||
The results showed that the A. paniculata extract was inactive against gram- negative bacteria Escherichia coli and gram- positive
bacteria Staphyloccocus aureus. Meropenem was used as positive
control. Meropenem is known for exhibiting strong antibacterial properties.
According to the legend above, results that are less than 10 mm are inactive,
10-13 mm is partially active, 14-19 mm is active, and greater than 19 mm is
very active. Three petri dishes were used for each bacterium. Both A. paniculata-AgNPs
produced at room temperature and at 60oC were partially active
against E. coli having a ![]() zone of inhibition of 12.15 mm and 11.06 mm,
respectively. Meanwhile, the synthesized AgNPs were active against S. aureus
with 14.38 mm
zone of inhibition of 12.15 mm and 11.06 mm,
respectively. Meanwhile, the synthesized AgNPs were active against S. aureus
with 14.38 mm ![]() mean zone of inhibition for A. paniculata-AgNPs
at room temperature and 14.18 mm for A.
paniculata-AgNPs at 60oC.
mean zone of inhibition for A. paniculata-AgNPs
at room temperature and 14.18 mm for A.
paniculata-AgNPs at 60oC.
Synthesized silver nanoparticles have shown superior
antimicrobial activity against all tested human pathogens among which exhibited
potent inhibitory activity against E.
coli and S. aureus.
6)
DPPH Radical Scavenging Assay
The antioxidant activity of the A. paniculata extract and A.
paniculata-AgNPs was assesses using DPPH
scavenging assay. Table 3 shows the dose
dependent increase in the inhibition percentage of the synthesized A.
paniculata-AgNPs (60oC) at 50, 100,
150, 200, 250, 300,350, 400 µL/mL concentration. As the concentration of A.
paniculata-AgNPs increases, the percentage
inhibition was found to be increase. Decreasing activity was observed with A. paniculata extract and A. paniculata-AgNPs
(room temp). In comparison to the extract, AgNPs have
shown less percentage of inhibition this may be due to the presence of less amount of functional groups adhered to the nanoparticles.
Table 3
|
Table 3 DPPH Radical Scavenging Assay Results |
|||
|
|
% Radical Scavenging Activity |
||
|
Concentration, µL/mL |
A. paniculata extract |
A. paniculata-AgNPs at room temp. |
A. paniculate-AgNPs at 60oC |
|
50 |
50.41 |
25.06 |
17.31 |
|
100 |
49.35 |
23.43 |
18.77 |
|
150 |
42.99 |
23.20 |
21.55 |
|
200 |
42.87 |
21.44 |
23.71 |
|
250 |
42.63 |
20.26 |
28.15 |
|
300 |
42.40 |
19.43 |
19.84 |
|
350 |
38.87 |
18.37 |
34.94 |
|
400 |
28.74 |
18.37 |
31.25 |
4. CONCLUSIONS and RECOMMENDATIONS
Synthesis of AgNPs capped with metabolites from A. paniculata leaves extract was confirmed by the color change from clear to yellowish brown which indicates formation of AgNPs. AgNPs are crystalline in nature as described by the diffraction pattern from XRD analysis. The EDX spectra of the sample showed that the silver and nitrogen were the most abundant element present in the samples. The SEM analysis revealed the surface morphology of and actual size of the AgNPs. It is concluded that synthesized AgNPs have antioxidant activity due to the capped metabolites. Also, the synthesized AgNPs have shown potential bacterial activity against human pathogenic bacteria. Synthesized AgNPs exhibited slightly equivalent antibacterial activity as compared to meropenem. These results suggest that in the future, AgNPs can be selected as potential antibacterial agent.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
The researcher would like to extend their gratitude to the Science Education Institute, Department of Science and Technology, the administration of Saint Mary’s University, and to Dr. Elsa Cajucom for the help in the completion of this study.
REFERENCES
Alharbi, N. S., Alsubhi, N. S., & Felimban, A. I. (2022). Green Synthesis of Silver Nanoparticles Using Medicinal Plants: Characterization and Application. Journal of Radiation Research and Applied Sciences, 15(3), 109-124. https://doi.org/10.1016/j.jrras.2022.06.012
Henglein, A. (1993). Physicochemical Properties of Small Metal Particles in Solution: 'Microelectrode' Reactions, Chemisorption, Composite Metal Particles, and the Atom-To-Metal Transition. The Journal of Physical Chemistry, 97(21), 5457-5471. https://doi.org/10.1021/j100123a004
Jayakumar, T., Hsieh, C. Y., Lee, J. J., & Sheu, J. R. (2013). Experimental and Clinical Pharmacology of Andrographis paniculataand its Major Bioactive Phytoconstituent Andrographolide. Evidence-Based Complementary and Alternative Medicine, 1-16. https://doi.org/10.1155/2013/846740
Keshari, A. K., Srivastava, R., Singh, P., Yadav, V. B., & Nath, G. (2020). Antioxidant and Antibacterial Activity of Silver Nanoparticles Synthesized by Cestrum Nocturnum. Journal of Ayurveda and Integrative Medicine, 11(1), 37-44. https://doi.org/10.1016/j.jaim.2017.11.003
Kumar, S., Singh, B., & Bajpai, V. (2021). Andrographis Paniculata (Burm.f.) Nees: Traditional Uses, Phytochemistry, Pharmacological Properties and Quality Control/Quality Assurance. Journal of Ethnopharmacology, 275. https://doi.org/10.1016/j.jep.2021.114054
Luhata, L.P., Chick, C.N., Mori, N., Tanaka, K., Uchida, H., Hayashita, T., & Usuki, T. (2022). Synthesis and Antioxidant Activity of Silver Nanoparticles Using the Odontonema strictum Leaf Extract. Molecules, 27(10), 3210. https://doi.org/10.3390/molecules27103210
Okhuarobo, A., Falodun, J. E., Erharuyi, O., Imieje, V., Falodun, A., & Langer, P. (2014). Harnessing the Medicinal Properties of Andrographis Paniculata for Diseases and Beyond: A Review of Its Phytochemistry and Pharmacology. Asian Pacific Journal of Tropical Disease, 4(3), 213-222. https://doi.org/10.1016/S2222-1808(14)60509-0
Shameli, K., Ahmad, M. B., Zargar, M., Yunus, W. M. Z. W., Rustaiyan, A. & Ibrahim, N. A. (2011). Synthesis of Silver Nanoparticles in Montmorillonite and their Antibacterial Behavior. International Journal of Nanomedicine, 581. https://doi.org/10.2147/IJN.S17112
Singh, J., Dutta, T., Kim, K.H., Rawat, M., Samddar, P., & Kumar, P. (2018). ‘Green’ Synthesis of Metals and their Oxide Nanoparticles: Applications for Environmental Remediation. J Nanobiotechnol 16, 84. https://doi.org/10.1186/s12951-018-0408-4
Song, J. Y., & Kim, B. S. (2008). Rapid Biological Synthesis of Silver Nanoparticles Using Plant Leaf Extracts. Bioprocess and Biosystems Engineering, 32(1), 79-84. https://doi.org/10.1007/s00449-008-0224-6
|
|
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© IJETMR 2014-2024. All Rights Reserved.


