|
|
|
|
New Investigation of Tooth Enamel’s Nanostructure via Cameraless T-ray Imaging Technique
1 Applied
Research & Photonics, Inc., 470 Friendship Road, Suite 10, Harrisburg, PA
17111, USA
|
|
ABSTRACT |
||
|
The
fascinating functions of biominerals such as tooth enamel are presumed to be
caused by the spatial and volumic organization of nanoscale building blocks
of biominerals. Tooth biominerals, primarily hydroxyapatite, organize
themselves in a very precise and hierarchical manner within the different
tooth tissues, viz., enamel, dentin, and cementum. This organization is
crucial for the tooth's strength, hardness, and overall function. Biominerals
organization involves self-assembly, interfacial organization, and others.
Here, we report results obtained from the new nonionizing, cameraless T-ray
imaging, accessing the sub-surfaces, allowing a new insight into the surface
and sub-surface nanostructures across a depth profile of a few millimeters.
The cameraless T-ray imaging demonstrates a great potential in nondestructive
bioimaging for different kinds of tissue samples including the hard tooth
tissue presented herein. |
|||
|
Received 04 February 2024 Accepted 03 March 2024 Published 18 March 2024 Corresponding Author Anis
Rahman, a.rahman@arphotonics.net
DOI 10.29121/ijetmr.v11.i3.2024.1416 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2024 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: T-Ray,
Cameraless Imaging, Human Tooth Enamel, Nanostructure, Noncontact |
|||
1. INTRODUCTION
The process of biomineralization involves production of minerals by living organisms, resulting in hardened or stiffened mineralized tissues Wikipedia article (n.d.) . The natural world is full of examples of biomineral formation including tooth enamel, seashells, carbonates in invertebrates, and calcium phosphates and carbonates in vertebrates, among others. These biominerals form structural features such as the bone and teeth in mammals and birds. Traditional and legacy tools for investigation of biomineral’s nanostructures include scanning electron microscopy (SEM), transmission electron microscopy (TEM), atomic force microscopy (AFM), and other legacy techniques. The main goal of characterizations is to be able to study and correlate the functionalities (e.g., of the tooth enamel) with the nanostructures. All the current art of the imaging instruments mentioned above, however, produce frozen-in-time images of a single surface of a given sample. It is of critical importance to inspect the interior (sub-surfaces) of biomaterials via a nondestructive and noninvasive route, but not available from the current art. Here, we describe a cameraless T-ray volume imaging technique for critical inspection of human tooth tissues, ex-vivo and ex-situ. T-ray is non-ionizing; hence it does not impart any radiation dose to human tissues. It is also able to penetrate both soft and hard tissues, thus providing a means of probing sub-surfaces in a nondestructive and noncontact manner.
Cameraless T-ray imaging with sub-nanometer resolution is a new technique that can be used to both visualize and quantify the nanostructures such as the tooth enamel of present investigation. While it has been applied to semiconductor and nanotechnology fields Rahman & Rahman (2019), Rahman (2023) to our knowledge no significant application for imaging tooth enamels’ sub-surface nanostructure via T-ray has been reported, nor any information similar to those reported here were revealed by other current techniques. The nanostructure imaging information obtained via T-ray route can be used to better understand how tooth enamel resists damage and potentially help the professionals with meaningful data for developing damage prevention routes such as caries and tooth decay. Dental caries, commonly known as cavities or tooth decay, is a condition in which the hard tissues of the teeth (enamel, dentin) progressively break down due to bacterial activity. Tooth enamel is the hardest substance in the human body. It is made up of tightly packed hydroxylapatite crystals, which give it its strength and durability. However, tooth enamel is also very vulnerable to damage from acids, bacteria, and abrasives. Often the cavity formation starts below the surface of a tooth which is not detectable at the early stage by the traditional X-ray and optical imaging. This is where the T-ray imaging technique is valuable. It can see below the surface and with sub-nanometer resolution it is able to inspect small caries on a layer-by-layer fasion at early stages.
Another reason why tooth enamels’ nanostructure via cameraless T-ray imaging is important is the fact that it can help one to understand the nature of tooth enamel wear and tear during normal activities. Additionally, the T-ray images can help one to identify early signs of tooth decay. It could also help a better understanding of the effects of different oral care products on tooth enamel, thus facilitating research efforts for development of more effective oral care options. Overall, cameraless T-ray imaging is a promising new technique that has the potential to revolutionize the understanding of tooth enamel and its role in oral health.
DeRocher et al. (2020) have investigated crystallites made of hydroxylapatite (Ca5(PO4)3(OH)), abbreviated as HAP, on the nanoscale. These HAP crystallites are the fundamental building blocks of enamel. The authors used a mechanical model based on density functional theory calculations. The authors reported that residual stresses on teeth enamel arise because of the chemical gradients. Their prediction from X-ray diffraction data is in agreement with preferential dissolution of the crystallite core in acidic media.
With the above review it is apparent that critical and accurate imaging for both the surface and sub-surfaces is important. However, all current techniques provide only a frozen-in-time image of a single surface at a time. This keeps from inspection of the intrinsic morphology of tooth enamel’s interior structure and composition in a nondestrctive fashion. Sectioning and polishing required for TEM imaging, for example, is not only time consuming and tedious, but the process may also perturb the structure. While X-ray allows to image the whole tooth, the interpretation of the interior is hindered by the wall shadows, thus creating complication. T-ray is the only means of non-ionizing energy that provides high-resolution images of tooth enamel, allowing one to see even the smallest details and sub-surfaces which may reveal microcavities and other structural defects. In what follows, we briefly describe the principle of cameraless T-ray imaging and high-resolution imaging with bigger wavelengths, followed by the imaging results of a human tooth. This has the potential to shed light in our understanding of tooth enamel and its role in oral health.
2. MATERIALS AND METHODS
2.1. Principle of camera-less T-ray imaging for nanoscale investigation
The principle of wavelength decoupled, high-resolution T-ray imaging without requiring a camera (i.e., a CCD or another kind of focal plane array) and overcoming the Abbe diffraction limit Abbe (1873) is described in detail elsewhere Rahman & Rahman (2019) in the context of semiconductors. As a non-contact technique, it is applicable to any material or a sample thereof. Here the technique is reviewed briefly.s
It is known that Abbe diffraction limit Abbe
(1873) sets a limt on
resolution for all imaging instrument by way of the wavelength dependence on
image formation physics. In the simplest form, the wavelength dependence is
expressed by ![]() ,
where
,
where ![]() is called the numerical aperture (NA). The
above-mentioned wavelength dependent resolution limitation is called the Abee’s
diffraction limit or simply diffraction limit, which indicates that smaller
wavelength is necessary for imaging smaller objects. This limitation eliminates
any bigger wavelength-based technology when it comes to image formation by a
lens-based system for imaging in the nanoscale and below, such as an atomic
lattice Novotny
& Hecht (2006). However, overcoming
the diffraction limit has become one of the primary goals of research in modern
optics. It has recently been demonstrated by Rahman et al. that nanoscale Rahman & Rahman (2019) and lattice resolution
imaging is possible with T-ray which is a bigger wavelength radiation. The
T-ray wavelength ranges from a few micrometers to a few thousand micrometers.
The authors devised a technique that eliminates the lens-based camera system
along with its image recording device such as a CCD, and hence the wavelength
dependence, by a nanoscanner system Rahman & Rahman (2019), Rahman (2023); where the spot size
of the incident beam is decoupled from the image resolution. Here a stratagem
has been devised that utilizes (1) a modified Beer-Lambert’s law written in
terms of the reflected intensity, (2) a nanoscanner that helps digitizing an object
over a user-specified volume, and storing the intensity in a 3-D matrix, termed
as the Beer-Lambert Reflection (BLR) matrix, and finally (3) an algorithm to
compute the image of the scanned volume from the measured BLR matrix Rahman & Rahman (2019). Among the many
advantages of this technique, non-destructive sub-surface inspection is
important. In addition, layer-by-layer inspection on all three orthogonal
directions with a resolution of £1 Å is
also important.
is called the numerical aperture (NA). The
above-mentioned wavelength dependent resolution limitation is called the Abee’s
diffraction limit or simply diffraction limit, which indicates that smaller
wavelength is necessary for imaging smaller objects. This limitation eliminates
any bigger wavelength-based technology when it comes to image formation by a
lens-based system for imaging in the nanoscale and below, such as an atomic
lattice Novotny
& Hecht (2006). However, overcoming
the diffraction limit has become one of the primary goals of research in modern
optics. It has recently been demonstrated by Rahman et al. that nanoscale Rahman & Rahman (2019) and lattice resolution
imaging is possible with T-ray which is a bigger wavelength radiation. The
T-ray wavelength ranges from a few micrometers to a few thousand micrometers.
The authors devised a technique that eliminates the lens-based camera system
along with its image recording device such as a CCD, and hence the wavelength
dependence, by a nanoscanner system Rahman & Rahman (2019), Rahman (2023); where the spot size
of the incident beam is decoupled from the image resolution. Here a stratagem
has been devised that utilizes (1) a modified Beer-Lambert’s law written in
terms of the reflected intensity, (2) a nanoscanner that helps digitizing an object
over a user-specified volume, and storing the intensity in a 3-D matrix, termed
as the Beer-Lambert Reflection (BLR) matrix, and finally (3) an algorithm to
compute the image of the scanned volume from the measured BLR matrix Rahman & Rahman (2019). Among the many
advantages of this technique, non-destructive sub-surface inspection is
important. In addition, layer-by-layer inspection on all three orthogonal
directions with a resolution of £1 Å is
also important.
2.2. Experimental
A clinically extracted human tooth (premolar) from a consenting patient was used for the present imaging investigations. The tooth was mounted on the nanoscanner as shown in Figure 1. The nanoscanner is a part of the instrument called TN3DI (Applied Research & Photonics, Inc., Harrisburg, PA), where a built-in front-end program was used to scan a small volume of one surface of the tooth. Subsequently, the scanned BLR matrix was used to generate images as discussed below. Sequential zooming was used to inspect and quantify different enamel properties. Additionally, a small volume of the same tooth sample was also scanned with appropriate scanning parameters for the generation of very high-resolution images.
Figure 1
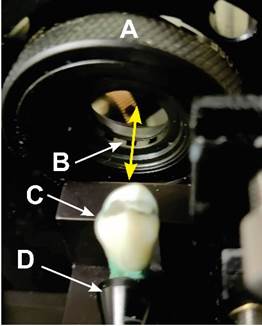
|
Figure
1 A Human Tooth Mounted on the
Nanoscanner. Measurements are Done in Reflection. A: T-Ray Beam Control
Aperture, B: T-ray, C: A Tooth, D: Tooth holder. |
3. RESULTS AND DISCUSSIONS
Figure 2
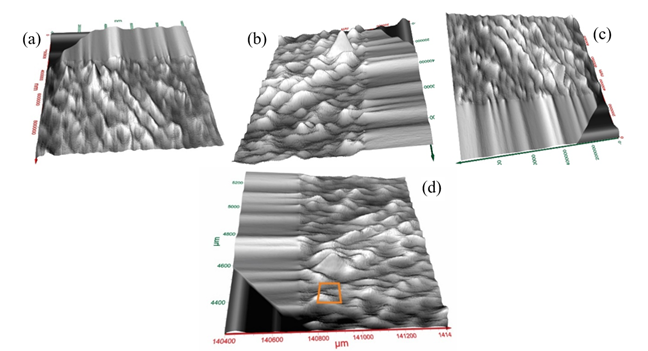
|
Figure
2 (a)-(d). Four Different Views
of T-Ray Surface Image of a Segment of the Tooth (1 mm × 1 mm) from Different
Viewing Angles |
Measurements on 1 mm × 1 mm × 0.1 mm volume from one side of the tooth were conducted. Surface images are shown in Figure 2 a-d from different viewing angles. Figure 3 displays a close-up view corresponding to the red square (100 µm × 100 µm) in Figure 2d. As seen from Figure 3, the image over the zoomed area of (100 µm)2, the arrangements of nanowire bundles are visible at the top and bottom sides of the image. The mid region of Figure 3(a) shows a different pattern due to the degradation of tooth enamel. Figure 3 (b) exhibts a close-up view of T-ray image corresponding to the red square in Figure 3 (a) showing the nano-wire bundles. These nanowires’ dimensions are measured.
Figure 3
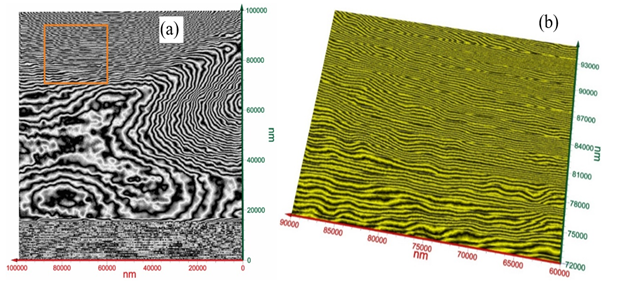
|
Figure 3 (a) Close-Up of T-Ray Image
Corresponding to the Red Square in Figure 2d Over 100 µm
× 100 µm Showing the Arrangements of Nanowire Bundles and the Mid Region with
Degradation. (b) Close-Up View of T-Ray Image Corresponding to the Red Square
in 3(a) Showing the Nano-Wire Bundles |
Figure 4 (a)-(b) exibit two different views of T-ray volume (3D) image (30 µm × 23 µm × 9 µm) of the tooth enamel of the present tooth sample. Figure 5 (a) exhibits a single surface of the image from Figure 4 (a) and Figure 5 (b) shows the graphical analysis of the nanowire bundles corresponding to the cursor in Figure 5 (a), see red arrow. The width of each nanowire is read off the graph (Figure 5 (b)). Average bundle size = Average (149, 94, 112, 93, 75, 93, 112, 131) = 107 nm at the location of cursor. However, there is significant variation of the nanowire bundles’ size, diameter, and orientations as depicted in Figure 5(a). Measurements may be conducted at any location of interest by a dental expert for the quantitative determination of the nanowire size.
Figure 4
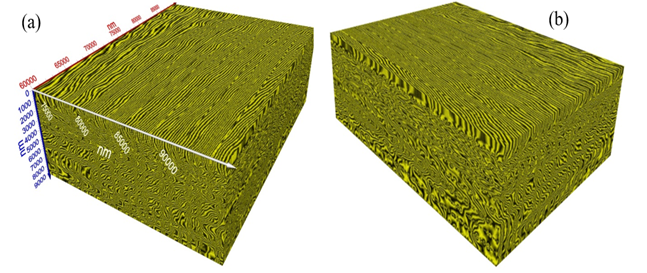
|
Figure
4 (a)-(b).
Close-Up Volume (3D) T-Ray Image (30 µm × 23 µm × 9 µm), Two Different Views. |
Figure 5
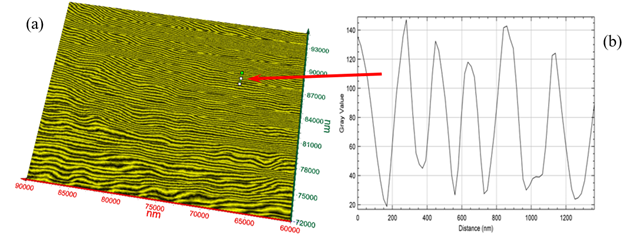
|
Figure
5 (a) T-Ray Image of Top Surface
from Figure 5 (b)
Graphical Analysis of the Nanowire Bundles. Average Bundle Size = Average
(149, 94, 112, 93, 75, 93, 112, 131) = 107 nm |
A special feature of the present T-ray volume imaging technique is the ability to slice the volume image into any number of slices for a closer inspection of the features, size, and defects, on a layer-by-layer basis along the three orthogonal axes. As an example of this, Figure 6(a) displays three different slices on the X-Y plane while Figure 6 (b) displays three slices on the Y-Z plane. The X-Z plane images are not shown here.
Figure 6
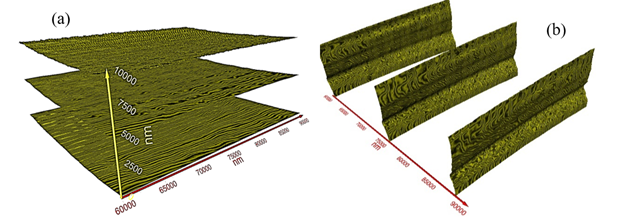
|
Figure
6 Three Different
Layers Extracted from Figure 4 (a) The X-Y Plane,
and (b) the Y-Z Plane. |
4. DISCUSSION and CONCLUSIONS
In this report we have demonstrated a new nanostructure imaging modality for dental enamel. It was found that the T-ray image reveals that a human premolar tooth’s interior surface, used for chewing food, exhibits significant roughness. There is a clear demarcation line that separates the inner surface near the gum line and the upper part of the tooth that is affected by chewing. Recently, there has been significant emphasis on the role of the nanowire bundle that forms the tooth enamel. Reports on Enamel crystallite strength and wear of nanoscale responses of teeth to chewing loads Xia et al. (2017), water-switchable interfacial bonding on tooth enamel surface Hua et al. (2018), and models for explaining tooth wear with implications for microwear formation Gowreesh et al. (2009), have elucidated different aspects of tooth wear, decay, defects, and their potential remedy. However, a nondestructive route for nanoscale imaging and quatifying the nanowire bundles have not been possible. Traditional transmission electron microscope requires sectioning and polishing, thus potentially changing the morphology of the sample. The scanning electron microscope produces only a surface image, not able to produce volume image. In all cases a frozen-in-time image is produced without the ability to analyze in a layer-by-layer fashion. Hence, the data presented herein is the first of its kind where a volumetric image of a human tooth enemel is generated via a nondestructive T-ray technique with layer-by-layer analysis option. This technique can be extended to any other tissues.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
Abbe, E. (1873). "Beiträge zur Theorie des Mikroskops und der mikroskopischen Wahrnehmung." Arch. Mikrosk. Anat. 9, 413–468. https://doi.org/10.1007/BF02956173
DeRocher, K. A., Smeets, P. J. M., Goodge, B. H., Zachman, M.J., Balachandran, P. V., Stegbauer, L., Cohen, M. J., Gordon, L. M., Rondinelli, J. M., Kourkoutis, L. F., & Joester, D. (2020). Chemical Gradients in Human Enamel Crystallites. Nature, 583, 66-71. https://doi.org/10.1038/s41586-020-2433-3
Gowreesh, S., Reddy, S.N., & Murthy, Y. N. V. (2009). Convective Heat Transfer Analysis of a Aero Gas Turbine Blade Using Ansys. International Journal of Mechanics and Solids. 4, 39-46.
Hua, L., Zheng, J., Zhou, Z., & Tian, Z.R. (2018). "Water-Switchable Interfacial Bonding on Tooth Enamel Surface." ACS Biomaterials Science & Engineering 4(7), 2364-2369. https://doi.org/10.1021/acsbiomaterials.8b00403
Novotny, L., & Hecht, B. (2006). "Propagation and Focusing of Optical Fields," in Principles of Nano-Optics, Cambridge U Press, 45-87. https://doi.org/10.1017/CBO9780511813535.004
Rahman, A. (2023). "T-Ray Wavelength Decoupled Imaging and Profile Mapping of a Whole Wafer for Die Sorting and Analysis," Sensors, 23. https://doi.org/10.3390/s23073663
Rahman, A., & Rahman, A. K. (2019). "Nanoscale Metrology of Line Patterns on Semiconductor by Continuous Wave Terahertz Multispectral Reconstructive 3D Imaging Overcoming the Abbe Diffraction Limit," in IEEE Transactions on Semiconductor Manufacturing. https://doi.org/10.1109/TSM.2018.2865167
Wikipedia Article (n.d.).
In Wikipedia.
Xia, J., Tian, Z. R., Hua, L., Chen, L., Zhou, Z., Qian, L., & Ungar, P. S. (2017). "Enamel Crystallite Strength and Wear: Nanoscale Responses of Teeth to Chewing Loads." Journal of the Royal Society Interface 14, 135. https://doi.org/10.1098/rsif.2017.0456
Xia, J., Zheng, J., Huang, D., Tian, Z. R., Chen, L., Zhou, Z., Ungar, P. S., & Qian, L. (2015). "New Model to Explain Tooth Wear with Implications for Microwear Formation and Diet Reconstruction." Proceedings of the National Academy of Sciences 112, 34, 10669-10672. https://doi.org/10.1073/pnas.1509491112
|
|
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© IJETMR 2014-2024. All Rights Reserved.


