|
|
|
|
PRODUCTION AND CHARACTERIZATION OF NANO-CACO3 FROM CLAMSHELL (Geloina sp.) BY TOP-DOWN METHOD
Anwar Maruf 1![]() , Achmad Basori 2
, Achmad Basori 2
1, 2 Chemical
Engineering Department of Universitas Muhammadiyah Purwokerto,
Indonesia
|
|
ABSTRACT |
||
|
The utilization of nano-CaCO3 is
currently growing very fast in various fields. The research aims to produce
and analyze the properties of
nano-CaCO3 from
clamshells (Geloina sp.). The production of nan-CaCO3 was
done by the top-down process using high-energy milling. The clamshell (Geloina sp.) is the potential resource of nano-CaCO3. The nano-CaCO3 can
be produced by the milling process. The main factor that affected the
yield is the number of steel balls, while the
speed of rotation and number of cycles have
a negative effect. The EDX analysis shows that the nano-CaCO3
has high
purity. The nano-CaCO3 from clamshell (Geloine sp.)
can be applicated as a drug delivery system and catalyst. |
|||
|
Received 14 September 2023 Accepted 15 October 2023 Published 31 October 2023 Corresponding Author Anwar
Maruf, anwarump@yahoo.com DOI 10.29121/ijetmr.v10.i10.2023.1374 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2023 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Nano-CaCO3,
Geloina sp., Top-Down Method |
|||
1. INTRODUCTION
The utilization of nano-CaCO3 is currently growing very fast in various fields. In the drug field, nano-CaCO3 is one of the intelligent carriers because of its biocompatibility and biodegradability, especially its sensitivity to pH Xing et al. (2020). The toothpaste that contains nano-CaCO3 can be used as an anticaries drug on the teeth Adnyani et al. (2020). In the industrial field, nano- CaCO3 can be used as a catalyst Mosaddegh et al. (2013). Nano-CaCO3 also can be used as a reliable, durable, and environment-friendly alternative to diminishing fly ash Poudyal et al. (2021). The nano- CaCO3 can also as an additive in the epoxy resin adhesive to increase the shear strength Kaybal et al. (2017). Nano- CaCO3 can also decrease the flowability and the setting time of fresh cement paste Liu et al. (2012).
Nano-CaCO3 can be made from chemicals and natural resources by several processes. From chemicals, nano- CaCO3 can be produced from CaO Adnyani et al. (2020) and CaCl2 Xing et al. (2020). From natural resources, nano- CaCO3 can be produced from materials that contain CaCO3 such as eggshells Mosaddegh et al. (2013), clamshells Widyastuti & Intan Ayu Kusuma (2017), pearl shells Wahyuningsih et al. (2019), and rocks Akhwady and Bayuaji (2017). Clamshell (Geloina sp.) is one abundance of natural resources of calcium carbonate (CaCO3). The production of Nano- CaCO3 from clamshells can be conducted by a top-down method and a bottom-up method. In the top-down method, the materials are milled until the nano-size is achieved Kamboj et al. (2020) Mosaddegh et al. (2013). While the bottom-up method, nano- CaCO3 can be produced by the precipitation process Ismail et al. (2022). The top-bottom method is simpler compared to the bottom-up method.
This study aims to produce nano- CaCO3 from clamshell by top-bottom method and characterize the nano- CaCO3 produced. Three main variables such as the number of balls, speed rotation, and the number of cycles is studied. The characteristics of nano- CaCO3 are studied using Fourier Transform Infra-Red (FTIR) and Scanning Electron Microscope (SEM).
2. MATERIALS AND METHODS
2.1. Materials
The Clamshell was obtained from the local market in the Cilacap Regency. The sodium hydroxide (NaOH) was obtained from Merck.
2.2. Preparation
The clamshell was washed and crushed until the particle size of 50 – 80 mesh. The powder of the clamshell was then dried at the temperature of 105oC. The clamshell powder was then deproteinized using NaOH solution Eke-Ejiofor and Moses (2019).
2.3. Milling Process
The milling process was using high-energy milling. High-energy milling was carried out with a number of steel balls of 20 with a diameter of 3 mm. The high-energy milling process uses a speed of rotation of 300 rpm and a number of cycles of 500,000 cycles. After the milling process, the milled CaCO3 was then sieved using a 500 mesh. The yield of the milling process was calculated using Eq. 1.
![]() Equation 1
Equation 1
where w0 is the weight of feed CaCO3 and w1 is the weight of nano-CaCO3. The design experiment is shown in Table 1.
Table 1
|
Table 1 Design Experiment |
||
|
|
Variables |
Value of Variables |
|
-1 |
1 |
|
|
Number of balls (X1) |
10 |
20 |
|
Speed of rotation (X2) |
300 |
400 |
|
Number of cycles (X3) |
5,00,000 |
10,00,000 |
Characterizations
The functional groups of nano- CaCO3 were studied using Fourier Transform Infra-Red (FTIR). The morphology of nano- CaCO3 was analyzed using Scanning Electron Microscope – Energy Dispersive X-Ray (SEM-EDX).
3. RESULTS AND DISCUSSIONS
3.1. Yield of Nano-CaCO3
Table 2 shows the yield of nano- CaCO3 obtained. The yields obtained are a range of 13.67 - 43.29%. Table 3 shows the effect of the main and interaction variables. Factorial design analysis shows that the main effect that effluence the yield is the number of balls (X1). While the most influential variable interaction is the interaction between the number of balls (X1) and the number of cycles (X3).
Table 2
|
Table 2 The
yield of nano-CaCO3 obtained |
||||
|
Run |
X1 |
X2 |
X3 |
Yield |
|
1 |
- |
- |
- |
40.95 |
|
2 |
+ |
- |
- |
34.85 |
|
3 |
+ |
+ |
- |
43.29 |
|
4 |
- |
+ |
- |
35.54 |
|
5 |
- |
- |
+ |
13.67 |
|
6 |
+ |
- |
+ |
40.84 |
|
7 |
+ |
+ |
+ |
15.42 |
Table 3
|
Table 3 Effect of variables |
|
|
Effects |
Values |
|
X1 |
8.85 |
|
X2 |
-0.67 |
|
X3 |
-49.31 |
|
X12 |
-64.13 |
|
X13 |
5.55 |
|
X23 |
-6.73 |
|
X123 |
-60.99 |
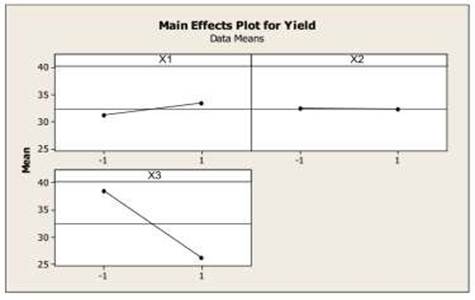
Figure 1 shows the main effects plot for yield. The number of steel balls has a positive effect (8.85), at a higher of a number of steel balls the yield of nano- CaCO3 obtained will be increased. The speed of rotation has a slightly negative effect (0.67), with the addition of the speed of rotation, the yield will decrease slightly. While the number of cycles has a negative effect (-49.31). The addition of the number of cycles will decrease of yield of nano- CaCO3. From the experimental data, it can be recommended that the best condition of nano- CaCO3 using high-energy milling is at the number of steel balls of 20, the speed of rotation of 300 rpm, and the number of cycles of 500,000 cycles.
Figure 1
|
Figure
1 Main Effects Plot of Process
Variables |
3.2. 3FTIR Spectrum of Nano- CaCO3
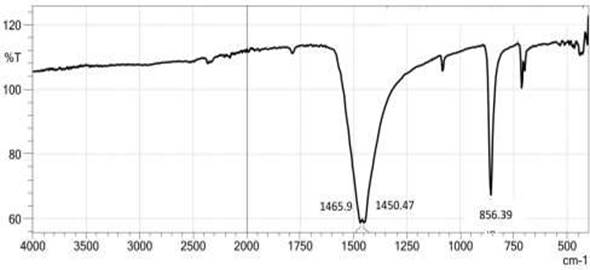
Figure 2 shows the FTIR spectrum of nano- CaCO3. There are three main peaks, respectively 1465.9, 1450.47, and 856.39 cm-1. The three peaks show the vibration of CO32- Ramasamy et al. (2017). Figure 3 shows the EDX analysis of nano- CaCO3. EDX analysis shows the chemical composition of nano- CaCO3. The mass percentage of Ca, C, and O are 28.15%, 17.28%, and 53.85% respectively. From the EDX analysis can be seen that nano-CaCO3 produced has high purity.
Figure 2
|
Figure
2 FTIR Spectrum
of CaCO3 |
Figure 3

|
Figure
3 EDX Analysis of Nano-CaCO3 |
3.3. Morphology of Nano-CaCO3
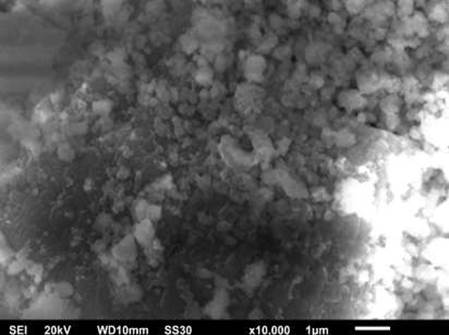
Figure 4 Show the morphology of nano- CaCO3 obtained from Geloina sp. Figure 3 shows that the nano- size was obtained during the milling process. The nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The bigger particle size occurred during the milling process because of the agglomeration of nano- CaCO3. This is following the results of the factorial analysis which shows that the number of cycles factor has a negative effect. The longer the cycle, the more particles agglomerate.
Figure 4
|
Figure
4 Morphology of Nano-CaCO3 |
4. CONCLUSIONS And RECOMMENDATIONS
The clamshell (Geloina sp,) is the potential resource of nano- CaCO3. The nano- CaCO3 can be produced by the milling process. The main factor that affected the yield is the number of balls, while the speed of rotation and number of cycles have a negative effect. The EDX analysis shows that the nano- CaCO3 has high purity. The nano- CaCO3 from clamshell (Geloine sp.) can be applicated as a drug delivery system and catalyst.
CONFLICT OF INTEREST
None.
ACKNOWLEDGMENT
The support of the Research and Community Service Board of Universitas Muhammadiyah Purwokerto is gratefully acknowledged (contract number: A.11-III/480-S.Pj./LPPM/XII/2021).
REFERENCES
Adnyani, N. M. L. G., Febrida, R., Karlina, E., Cahyanto, A., and Made Joni, I. (2020). Synthesis of Nano Calcium Carbonate from Natural CaO by CO2 fine bubbling method. AIP Conference Proceedings, 2219. https://doi.org/10.1063/5.0003072.
Akhwady, R., and Bayuaji, R. (2017). The Influence of Clamshell on Mechanical Properties of Non-Structure Concrete as Artificial Reef. In Asian Journal of Applied Sciences. https://doi.org/10.24203/ajas.v5i2.4620.
Eke-Ejiofor, J., and Moses, R. E. (2019). Preparation and Evaluation of Food Grade Preservatives from Shells of Locally Available Shellfishes. International Journal of Biotechnology and Food Sciences, 7(2), 23–29. https://doi.org/10.33495/ijbfs_v7i2.19.103.
Ismail, R., Cionita, T., Shing, W. L., Fitriyana, D. F., Siregar, J. P., Bayuseno, A. P., Nugraha, F. W., Muhamadin, R. C., Junid, R., and Endot, N. A. (2022). Synthesis and Characterization of Calcium Carbonate Obtained from Green Mussel and Crab Shells as a Biomaterials Candidate. Materials, 15(16). https://doi.org/10.3390/ma15165712.
Kamboj, A., Amjad, M., Ahmad, W., & Singh, A. (2020). A General Survey on Green Synthesis and Application of Calcium Oxide Nanoparticles. International Journal of Health and Clinical Research, 3(2), 41–48.
Kaybal, H. B., Ulus, H., and Avci, A. (2017). Influence of Nano-CaCO3 Particles on Shear Strength of Epoxy Resin Adhesives. Uluslararası Muhendislik Arastirma ve Gelistirme Dergisi, 29–35. https://doi.org/10.29137/umagd.371119.
Liu, X., Chen, L., Liu, A., and Wang, X. (2012). Effect of Nano-CaCO3 on Properties of Cement Paste. Energy Procedia, 16(PART B), 991–996. https://doi.org/10.1016/j.egypro.2012.01.158.
Mosaddegh, E., Hassankhani, A., Pourahmadi, S., and Ghazanfari, D. (2013). Ball Mill-Assisted Preparation of Nano-CaCO3 as a Novel and Green Catalyst-Based Eggshell Waste : A Green Approach in the Synthesis of Pyrano[4,3-b] Pyrans. International Journal of Green Nanotechnology, 5(1), 1–5. https://doi.org/10.1177/1943089213507160.
Poudyal, L., Adhikari, K., & Won, M. (2021). Nano Calcium Carbonate (CaCO3) as a Reliable, Durable, and Environment-Friendly Alternative to Diminishing Fly Ash. Materials, 14(13). https://doi.org/10.3390/ma14133729.
Ramasamy, V., Anand, P., and Suresh, G. (2017). Biomimetic Synthesis and Characterization of Precipitated Caco3 Nanoparticles Using Different Natural Carbonate Sources : A Novel Approach. In International Journal of Materials Science, 12(3).
Wahyuningsih, K., Jumeri, and Wagiman. (2019). Optimization of Production Process of Nano- Calcium Oxide from Pinctada Maxima Shell by Using Taguchi Method. Indonesian Journal of Chemistry, 19(2), 356–367. https://doi.org/10.22146/ijc.33871.
Widyastuti, S., & Intan Ayu Kusuma, P. (2017). Synthesis and Characterization of CaCO3 (calcite) Nano Particles from Cockle Shells (Anadara Granosa Linn) by Precipitation Method. AIP Conference Proceedings, 1855. https://doi.org/10.1063/1.4985488.
Xing, J., Cai, Y., Wang, Y., Zheng, H., and Liu, Y. (2020). Synthesis of Polymer Assembled Mesoporous CaCO3 Nanoparticles for Molecular Targeting and pH-Responsive Controlled Drug Release. Advances in Polymer Technology, 2020. https://doi.org/10.1155/2020/8749238.
|
|
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© IJETMR 2014-2023. All Rights Reserved.


