|
|
|
|
Co-Solvent Tetrahydrofuran in the Production of Biodiesel from Used cooking oil Using the Transesterification Method
Robiah 1, Marhaini 1![]() , Amrina
Rosyada 1
, Amrina
Rosyada 1
1 Chemical Engineering Study Program,
Faculty of Engineering, Muhammadiyah University of Palembang, Indonesia
|
|
ABSTRACT |
||
|
Used cooking oil is a waste oil derived from cooking oil that has the potential to be produced into biodiesel because it contains fatty acids and triglycerides like other vegetable oils, however, its utilization is still low. This study aimed to analyze the effect of co-solvent THF based on reaction time and oil: methanol molar ratio in the manufacture of biodiesel using used cooking oil as raw material. Transesterification is a method used in biodiesel production by reacting raw materials with alcohol. KOH1% is used as a catalyst in the process of converting used cooking oil into biodiesel. In its operation, reaction time variations of 5, 7, 10, 13, and 15 minutes and molar ratios of 1:3, 1:6, 1:9, 1:12, and 1:15 were used at a temperature of 65oC. The results showed that when entering the reaction time of 10 minutes the density and viscosity tend to be stable and meet the standards of SNI 7182:2015, namely the density is stable at 0.88 gr/ml and the viscosity is in the range of 4.6238 cSt-5.7336 cSt. Meanwhile, based on the molar ratio, it was obtained only at a molar ratio of 1:6 which is in accordance with the characteristics of biodiesel based on SNI 7182:2015 which has a density of 0.88 gr/ml and a viscosity of 4.6238 cSt. The best % yield was at a 1:6 molar ratio with a reaction time of 13 minutes, that is 88.8199% with a heating value of 33.0398 MJ/kg and a cetane number of 66.9. Based on the methyl ester test with GC-MS, the methyl ester (biodiesel) content in the product was 87.24%, dominated by Oleaic (9(Z)-Octadecenoic) acid or 49.33% oleic acid and Palmitic (Hexadecanoic) acid. or palmitic acid by 34.08%. |
|||
|
Received 17 January 2023 Accepted 16 February 2023 Published 01 March 2023 Corresponding Author Marhaini, marhainiump@gmail.com DOI 10.29121/ijetmr.v10.i3.2023.1274
Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2023 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Used Cooking
Oil, Co-Solvent THF, Transesterification, Biodiesel |
|||
1. INTRODUCTION
The utilization of used cooking oil as raw material for
biodiesel is currently not thoughtfully developed in Indonesia. In fact, a
study by Traction Energy Asia 2019 shows that of the 28.4 kilolitres (KL) of
used cooking oil produced in Indonesia, the potential for biodiesel is 5.7 KL litres
Traction
Energy Asia. (2019). This potential may
meet the national biodiesel needs of up to 32%. However, only 3 million KL of
used cooking oil has been collected in Indonesia, so only 570 thousand KL is
used as biodiesel. The remaining 2.43 million KL is produced as a recycled cooking
oil and 184 thousand KL is exported Aprobi (2022).However, used cooking oil has high levels of FFA,
this is due to the formation of free fatty acids in used cooking oil that
occurs during frying so that a hydrolysis reaction occurs in triglycerides,
producing free fatty acids, diglycerides, monoglycerides, and glycerol which
can be indicated from the acid number Setyawati and Edwar
(2012). This condition may interfere with the
biodiesel production process because the transesterification process uses an
alkaline catalyst which if it reacts with free fatty acids it will experience
saponification so it needs to be pre-treated on the raw materials. In this
study, the handling of FFA uses an adsorbent from activated charcoal from palm
oil shells, this refers to the study of Fadillah et
al. (2017) which found that adsorbents from activated charcoal
from oil palm shells had reduced effectiveness of 91.82%, this percentage is
significant compared to coir-rice husk which is 57.06% and activated charcoal
from coconut shell by 33.33%.Transesterification is a slow reaction process because it takes place
through a two-phase system. This can be overcome by adding unreactive
co-solvent. The purpose of adding co-solvent is to form a solution system from
two phases into one phase. The use of Co-solvent THF is to overcome the
difference in solubility, in this case, THF is the best co-solvent; cheap,
non-reactive, low boiling point (67oC), and non-toxic, therefore it
can be separated by co-distillation with methanol and can be recycled at the end
of the reaction Sidi et al. (1996). THF also
has hydraulic and hydrophobic properties and can bind water and alcohol for its
hydrophilicity, while its hydrophobicity can dissolve organic compounds Ontario et al. (2003). In this study, the addition
of Co-solvent THF in the transesterification reaction of making biodiesel from
used cooking oil is strongly influenced by the reaction and the molar ratio of
the resulting product.
2. MATERIAL AND METHOD OF STUDY
2.1. MATERIALS AND TOOLS
Three neck flasks, return cooler, magnetic stirrer,
thermometer, a set of distillation apparatus, cooler, stopwatch, Pyrex brand of
glassware, hotplate, filter paper, separating funnel, analytical balance, oven,
Otswald viscometer, and pycnometer. Used cooking oil,
potassium hydroxide, methanol, tetrahydrofuran, palm shell activated charcoal, demineralized
water, and phenolphthalein indicator.
2.2. PROCEDURE OF STUDY
2.2.1. PRETREATMENTOF USED COOKING OIL
The raw
material of used cooking oil was obtained from mobile fried food vendors. Free Fatty
Acid (FFA) levels were reduced by being adsorbed for 24 hours with 35 mesh palm
shell activated carbon, then titration was carried out to determine the FFA
levels in the sample using a titrant of 0.1 N KOH solution and the use of
phenolphthalein as an indicator. If the FFA content is less than 0.5% then the
raw material could be continued to the transesterification stage.
2.2.2. TRANSESTERIFICATION STAGE
The
temperature of the used cooking oil was increased to 60oC, after the
temperature was reached, the oil was contacted with methanol, KOH catalyst, and
Co-solvent THF which had been mixed in a separate container. The
transesterification was carried out under 65oC operating conditions,
1%-wt KOH catalyst from oil, co-solvent 1:1% vol
methanol, and stirring speed of 75-150 rpm. The molar ratio of oil: methanol =
1: 6 at various times of 5, 7, 10, 13, and 15 minutes. The best reaction time
is indicated by the highest % yield and characteristics according to SNI
7182:2015, the best reaction time is used as reaction time at molar ratio of
1:3, 1:6, 1:9, 1:12, and 1:15.
3. RESULT AND DISCUSSION
3.1. EFFECT OF ACTIVATED CARBON ON FREE FATTY ACID LEVELS
In the pretreatment of used cooking oil derived from crispy fried foods
at mobile fried food vendors, the free fatty acid content was reduced by the
adsorption method using activated charcoal from palm oil shells. The temperature used for
frying is not too high for a relatively short time so that the free fatty
acid content formed is slight at 1.130%. After going through adsorption, the FFA
content decreased to 0.512%. FFA reduction by adsorption method is more
economical and easier and more effective than FFA reduction by esterification
using acid and methanol catalysts Usman et al. (2019). Oil with 0.512% FFA content has met the
requirements for the transesterification stage.
3.2. EFFECT OF REACTION TIME AND MOLAR RATIO ON % YIELD
3.2.1. EFFECT OF REACTION TIME ON % YIELD
Observation of
the effect of reaction time on % yield of biodiesel produced from the
transesterification process with fixed variable operating conditions; amount of
feed (70 ml), reaction temperature (65oC), stirring speed (75-150
rpm), molar ratio of oil and methanol (1:6), amount of co-solvent (1:1 %vol
methanol), weight of catalyst (1 %wt of oil), while the variable is the
reaction time (5, 7, 10, 13, and 15 minutes).
Figure 1
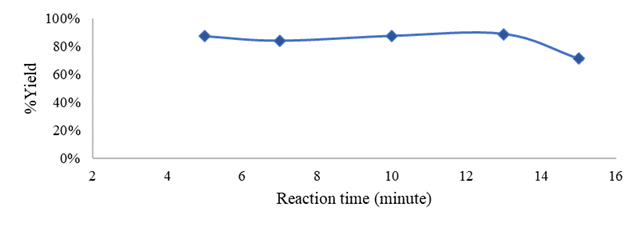
|
Figure
1 Effect
of Reaction Time on % Yield |
Figure 1 shows that % yield tends to increase slightly with increasing reaction
time. The addition of time provides more
opportunities for collisions between reactants so it gives more opportunities to
react. The more volume of product produced, the % yield increases. After the
best reaction time was reached, the % yield began to decrease, and an
equilibrium occurred, so the addition of reaction time exceeding 13 minutes
resulted in a decrease in % yield. The decrease occurs because the
transesterification reaction is a reversible reaction so when the reaction has
reached equilibrium, the addition of reaction time causes the reaction to shift
towards the reactants (to the left) and results in a decrease in % yield
(Setyawati et al, 2012). At the reaction time of 5 minutes, the yield reached
87.1429%, while at the reaction time of 7 minutes the yield decreased to
84.0373% and the yield increased again to 87.4534% in 10 minutes, this is
because, at the reaction time of 5 minutes the cooking oil has not been used
yet completely converted into biodiesel, this refers to the characteristics of
biodiesel such as the amount of density and viscosity. The product densityat 5
minutes has the same density as the raw material, which is 0.92 g/ml and has a
high viscosity of 14.9928 CST. Thus, at the reaction time of 5 minutes, the raw
material has not been properly converted into biodiesel. However, at a reaction
time of 7 minutes, the density and viscosity of the biodiesel product were in
accordance with SNI 7182:2015, so it could be said to be well converted, where
% Yield continued to increase at a reaction time of 13 minutes until it reached
88.8199%, but at 15 minutes conversion decreased to 71.2733%. The best reaction
time was obtained at 13 minutes and the co-solvent was very influential
compared to without the use of co-solvent, which generally required a minimum
of 1 hour to achieve the same yield.
3.2.2. EFFECT OF MOLAR RATIO ON % YIELD
Observations on
the effect of the molar ratio of reactants on % yield of biodiesel produced in
the transesterification process, the fixed variables used were the best
reaction time taken from the previous data (13 minutes), reaction temperature
(65oC), amount of feed (70 ml), stirring speed ( 75-150 rpm), the weight of
catalyst (1%wt of oil), and amount of co-solvent (1:1% vol methanol).
Meanwhile, the independent variable is the molar ratio of oil and methanol,
namely 1:3, 1:6, 1:9, 1:12, and 1:15.
Figure 2
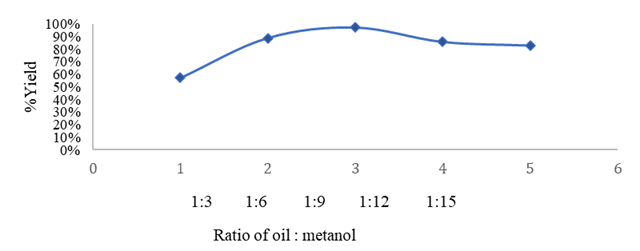
|
Figure
2 Effect of Molar Ratio on %
Yield |
Figure 2 shows a significant increase in yield with an increase in the ratio of reactants to 1:9. In a reversible reaction, the addition of excess methanol will push the reaction to the right, so that the conversion of oil into biodiesel increases Razak et al (2015). In this study, the highest yield was obtained at the oil: methanol ratio of 1:9 with a yield of 97.1429%, then the graph decreased at the molar ratio of 1:12 and 1:15. The transesterification reaction is an equilibrium reaction so that if equilibrium has been reached, the addition of moles of methanol and the reaction time does not affect the increase in yield of methyl ester. Excessive use of methanol will cause an increase in the formation of glycerol in proportion to the formation of methyl esters (biodiesel), but glycerol will dissolve in excess methanol, making it difficult to separate biodiesel and glycerol Zukhriyah et al. (2022).
3.3. EFFECT OF REACTION TIME AND MOLAR RATIO ON BIODIESEL CHARACTERISTICS
3.3.1. EFFECT OF REACTION TIMEON DENSITY
Figure 3
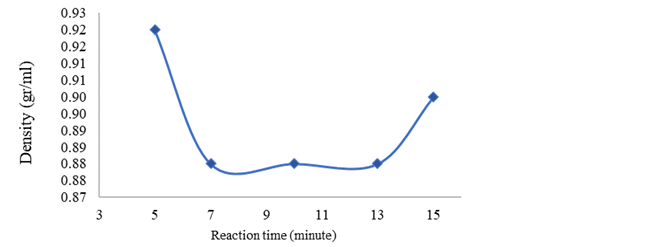
|
Figure
3 Effect of Reaction Time on Density |
Figure 3 shows the relationship of the effect of
reaction time on product density. It can be explained that at the beginning of
the treatment the density was still too high, similar to the density of the raw
material, which was 0.92 gr/ml, but at reaction times of 7, 10, and 13 minutes
the density was consistent at 0.88 gr/ml which complied with SNI 7182:2015
biodiesel. At the reaction time of 15 minutes the density increased to 0.90
g/ml, this was related to a decreased yield, allowing the presence of other
compounds such as the length of the carbon chain, therefore the greater the
number of double bonds, the higher the density value of biodiesel Yanowitz et al. (2016). However, the accuracy of the balance used is
only 1 digit behind the comma, making it difficult to read the weight, but the
density of this product is close to SNI 7182:2015 biodiesel. Thus the biodiesel
obtained has met the biodiesel density standard.
3.3.2. RELATIONSHIP BETWEEN MOLAR RATIO AND PRODUCT DENSITY
Figure 4 Shows that the product that meets
the quality requirements of SNI 7182:2015 is only at a ratio of 1: 6, which is
0.88 gr/ml. This is because the use of too little methanol (1 : 3) has not been
able to convert the raw material into biodiesel, so it has similar
characteristics to the raw material, namely 0.92 g/ml, while the product has a
larger density of 0.92. gr/ml has an indication that the reaction is not
perfect in the conversion of oil. This condition corresponds to the % yield
obtained which allows the presence of impurities in the product, such as
residual catalyst and methanol, glycerol, soap, water, and fatty acids that are
not converted to methyl esters or other content in the product that can affect
the density of the product. biodiesel Setyawati and Edwar
(2012).
Figure
4
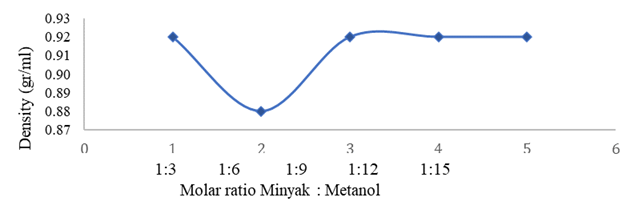
|
Figure
4 Effect of Molar Ratio on Density |
3.3.3.
REACTION
TIME TO PRODUCT VISCOSITY
Figure 5 shows that in this study kinematic
viscosity was found. The value of kinematic viscosity tends to decrease with
the length of reaction time. According to Setyawati and Edwar
(2012), the length of reaction time indicates
that the resulting product has a mixed composition consisting of compounds with
a smaller number of carbons Setyawati and Edwar
(2012).
Figure 5
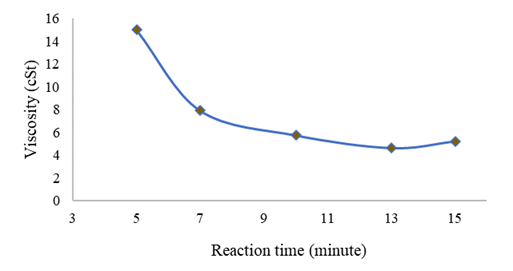
|
Figure
5 Effect of Reaction Time on Viscosity |
At the reaction time
of 5 minutes, the conversion was not perfect so the viscosity value was high
(14.9928 cSt), but at the reaction time of 10 to 15 minutes, the viscosity
value met the requirements and quality of SNI biodiesel of 4.6238-5.7336 cSt.
The test results showed that the biodiesel yield in the transesterification process
at 10, 13, and 15 minutes was in the range of 2.3 - 6.0 mm²/s. This indicates
that some biodiesel produced are in accordance with the viscosity value
according to the SNI 7182:2015 standard.
3.3.4. RELATIONSHIP BETWEEN MOLAR RATIO AND PRODUCT
VISCOSITY
Figure 6 shows the molar ratio to viscosity.
It can be observed that the viscosity value is in the range of SNI 7182:2015
biodiesel, this indicates that at a reaction time of 13 minutes, the variation
of the methanol molar ratio tends to be stable, except in the 1:3 molar ratio
the mixture viscosity of 7.5840 cSt exceeds the
biodiesel standard, this is due to these conditions the yield has only reached
57.1429% so that the product mixture still contains a lot of triglycerides.
Figure 6
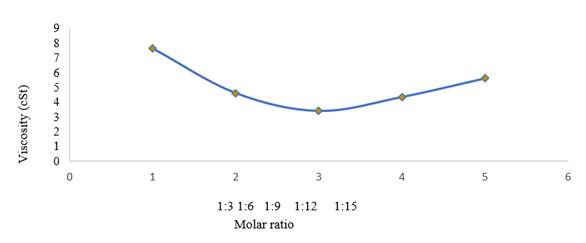
|
Figure
6 Effect of Molar Ratio on Viscosity |
Viscosity is a
very important property in the storage and the use of fuel. Too high viscosity affects the performance of the fuel
injection device and makes it difficult to ignite, causing poor atomization and
increasing engine deposits Setyawati and Edwar
(2012). However, too low a viscosity can also
result in a leak in the fuel injection pump. The viscosity of the fuel also has
a direct effect on the ability of the fuel to mix with air, causing incomplete
combustion.
3.3.5. EFFECT OF REACTION TIME AND MOLAR RATIO ON MOISTURE CONTENT
In the process
of making biodiesel, it allows for the emergence of a mixture of water. This
mixed water may come from raw materials, catalysts, and during the purification
process. However, the amount of water in the raw material (used cooking oil) is
very small due to the hydrolysis reaction when frying repeatedly in cooking
oil, thus breaking down H2O compounds and reacting with other
compounds to form new compounds. KOH catalyst has hygroscopic properties so it
has a good ability to absorb water molecules when exposed in the open Sangha et al. (2005). In the purification process,
demineralized water is used as the purification medium, so the possibility of
too fast separation between biodiesel and purified water will result in a large
amount of water in biodiesel. Therefore, at the end of the purification stage,
the product is dried by heating it at a temperature of 110oC to
evaporate the remaining water in the product.
Figure
7
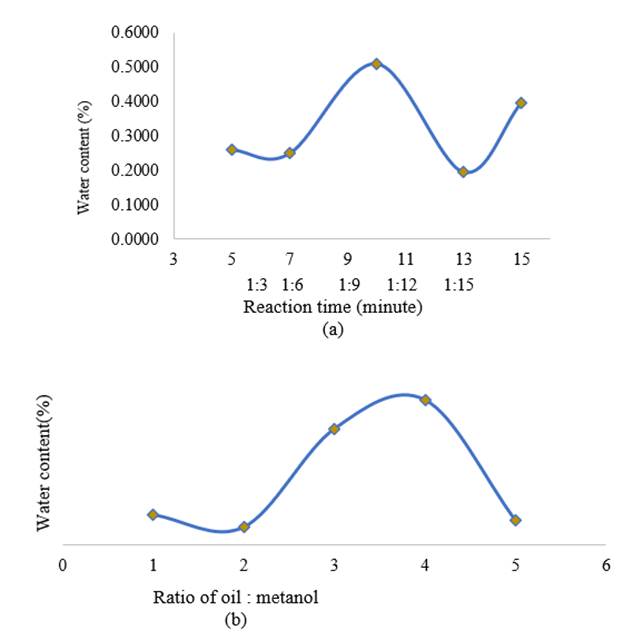
|
Figure
7 (a) Effect of Reaction Time on Water
Content (b) Effect of Molar Ratio
on Water Content |
Figure 7 shows the relatively high-water content. The standard water content allowed for biodiesel is a maximum of 0.05%. The high-water content is caused by several technical errors such as poor separation during the washing process of biodiesel with demineralized water, drying biodiesel that is not long enough so that the water content is still high in biodiesel, incomplete drying of biodiesel products that have gone through the washing process, and a balance with high accuracy is required for the accuracy of weighing the product.
Water can exist in free form and can cause damage to the
interior of the furnace surface during combustion especially if it contains
dissolved salts. Water can also cause sparks at the tip of the burner which can
extinguish the flame, lower the flame temperature, or prolong ignition. However, water content is not a big problem in
diesel vehicles that have a water sediment filter. Water sediment has a
function as a filter to separate water from fuel, so that diesel oil entering
the combustion chamber remains free of water (Final B30, Ministry of Energy and
Mineral Resources, 2020).
3.4. FLASH POINT AT MOLAR RATIO OF 1:6 AND REACTION TIME OF 13 MINUTES
The flash point
of biodiesel according to SNI is at least 100 oC and based on the
Decree of the Director General of EBTKE No.189/2019 the flash point of
biodiesel is at least 130oC, so it is within safe limits against
fire hazards while in storage, handling, and transportation below that
temperature. Based on the data of the study, the flash point is 178.3oC,
this number is a safe number because it is in accordance with the standards and
quality of biodiesel, so it is safe in handling and storage. The more water
content in the fuel, the more energy needed to evaporate the water so that the
flash point will be higher, and vice versa Fadilah
et al. (2019).
3.5. CALORIFIC VALUE AT MOLAR RATIO OF 1:6 AND REACTION TIME OF 13 MINUTES
In the
calorific value test, it was found that the calorific value under the best
conditions (molar ratio 1:6 and reaction time 13 minutes) was 7.891.4335 Cal/gr
or equivalent to 33.0398 MJ/kg while the calorific value of diesel fuel
(diesel) which is 44 MJ/kg. This low calorific value can be caused because
biodiesel does not contain aromatic compounds like diesel fuel, but methyl
esters with different levels of saturation. Unsaturated esters have lower
energy content by weight but because of their higher density, they have more
energy per unit volume Wahyuni et al. (2011). The amount of unsaturated ester content
has a calorific value that is not too high, the composition of this ester can
be identified by GC-MS testing.
3.6. CETANE NUMBER OF BIODIESEL PRODUCTS
The cetane
number can be shown by itself in the diesel engine combustion chamber when the
fuel ignites, this is with a high cetane number there will be an acceleration
of combustion and the thermodynamic efficiency is get better.
The cetane
number test was carried out on biodiesel products under the best conditions,
the biodiesel cetane number was 66.9. This figure shows the feasibility of the
product to be used as diesel fuel because this figure has met the SNI
biodiesel, which is at least 51. The cetane number obtained is also equivalent
to previous studies where biodiesel has a cetane number of 62 for palm
oil-based biodiesel, 51 for Jatropha, and 62.7 for coconut-based vegetables Soerawidjaja (2003).
The
composition of fatty acids in biodiesel, such as long-chain saturated fatty
acids (arachidic, lauric, myristic, palmitic, and lauric acids), is a high
cetane number composition. Thus, the fatty acid composition is closely related
to the cetane number of biodiesels. The cetane number has a significant effect
on timing, on the fuel injected, and will cause a smooth engine noise and a
good start.
3.7. COMPOSITION OF CHEMICAL COMPOUNDS IN TESTING USING GC-MS
Composition
testing with GC-MS was carried out at a 1:6 molar ratio and a reaction time of
13 minutes. It was obtained that the resulting product contains the most methyl
esters at 87.24%, while other compounds are 12.76%. This proves that the
resulting product is biodiesel due to its high methyl ester content. The
composition of this biodiesel product is shown in Figure 8. Figure 8. shows the readings of the composition of
the methyl ester types tabulated in Table 1. Results of the GC-MS show that
there are 2 main peaks that have the largest content in the product
composition, namely Oleaic (9(Z)-Octadecenoic) acid or oleic acid of 49, 33% at
a retention time of 14,589 minutes and Palmitic (Hexadecanoic) acid or palmitic
acid of 34.08% at a retention time of 13,443 minutes, the composition of these
compounds affects the high cetane number in the product.
Figure 8
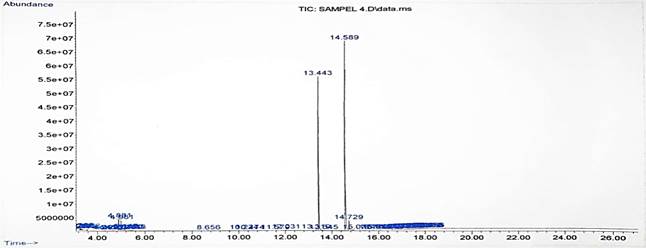
|
Figure 8 Results Of GC-MS Test on Biodiesel Products With 1:6 Molar Ratio at 13 Minutes Reaction Time |
The methyl
ester content affects the measurement of the calorific value and cetane number
of biodiesel products, where oleic acid is an unsaturated fatty acid, so it has
a low calorific value but the content of palmitic acid which is a saturated
fatty acid in principle has a high calorific value which can balance the
calorific value so it does not too low.
Table 1
|
Table 1 Data of GC-MS Analysis Results |
|
|
Component |
Compound
Composition (%) |
|
Metil ester: |
|
|
Oleaic (9(Z)-Octadecenoic) acid |
49,33 |
|
Palmitic (Hexadecanoic) acid |
34,08 |
|
Stearic (isooctadecanoic) acid |
3,44 |
|
Myristic (tetradecanoate) acid |
0,16 |
|
Palmitoleic(9(Z)-Hexadecanoic) acid |
0,18 |
|
Lauric (dodecanoic) acid |
0,05 |
|
Other compound |
12,76 |
|
Total |
100 |
In this study,
the biodiesel produced has a calorific value of 7,891.4335 cal/gr. Referring to
the research of McCormick et al. (2001) which measured the cetane number of oleic acid
methyl ester that the value was 59.3, while palmitic acid was measured in the
study of Knothe et al. (2005), the CN was 74.5. This figure corresponds
to the measurement of the CN biodiesel in this study, which is 66.9.
4. CONCLUSION
Co-solvent Tetrahydrofuran was very
influential in the manufacture of biodiesel in shortening the time, biodiesel
has started to convert well at a reaction time of 10 minutes. Meanwhile,
transesterification without the use of co-solvent takes a long time (+1 hour)
to convert raw materials into biodiesel products.
Based on the
effect of reaction time, it is concluded that the longer the reaction time, the
greater the conversion. The best reaction time is at a reaction time of 13
minutes with a %yield of 88.8199%. When entering the reaction time of 10
minutes the density and viscosity tend to be stable and meet SNI 7182:2015 with
a stable density at 0.88 gr/ml and viscosity in the range of 4.6238 cSt-5.7336
cSt. Meanwhile, based on the molar ratio of density and stable viscosity, it is
obtained only at a molar ratio of 1:6 which is in accordance with the
characteristics of biodiesel based on SNI 7182:2015 which has a density of 0.88
gr/ml and a viscosity of 4.6238 cSt.
Based on the GC-MS test, the methyl ester content in biodiesel products was 87.24%, dominated by Oleaic (9(Z)-Octadecenoic) acid or oleic acid at 49.33% and Palmitic (Hexadecanoic) acid or palmitic acid of 34.08%.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
Alleman,
T. L., Mccormick, R. L., Christensen, E. D., Fioroni, G., Moriarty, K., and
Yanowitz, J. (2016). Biodiesel Handling and Use Guide (5th Ed). National
Renewable Energy Laboratory. https://doi.org/10.2172/1332064.
Aprobi. (2022). Pemerintah Perlu Serius Memaksimalkan Used Cooking Oil Sebagai Biodiesel. Tribunnews.Com. (Diakses. Juni 20, 2022).
Arfah, M., and Razak. (2015). Optimasi Reaksi Esterifikasi Asam Laurat dengan Metanol Menggunakan Katalis Asam Sulfat Pekat. Online. Natural Science, 4(1), 46-55.
Boocock, D. G. B., 24 Bolland Crescent. (2003). Single-Phase Process for Producyion of Fatty Acid Methyl Esters Frommixture Triglyseides and Fatty Acids. LIS. Verlag 3G7.
Boocock, D. G. B., Konar, S. K., Mao, V., and Sidi, H. (1996). Fast One-Phase Oil-Rich Process for the Preparation of Vegetable Oil Methyl Esters. Biomass and Bioenergy, 11, 43-50. https://doi.org/10.1016/0961-9534(95)00111-5.
Dahlia, N., Rahmalia, W., and Usman, T. (2019). Adsorpsi
Asam Lemak Bebas Pada Crude Palm Oil Menggunakan Zeolit Teraktivasi K2CO3.
Indonesian Journal of Pure and Applied Chemistry, 2(3), 112-120. https://doi.org/10.26418/indonesian.v2i3.36892.
Daryono, E. D., Rahman, F. F. A., and Zukhriyah. (2022). Penggunaan Metanol Sisa Reaksi Sebagai Reaktan Pada Proses Transesterifikasi Minyak Kelapa Sawit Menjadi Biodiesel. Jurnal Teknologi. Universitas Muhammadiyah Jakarta, 14, No.2.
Elfian, F. (2017). Adsorpsi Arang Aktif Cangkang Kelapa Sawit Terhadap Warna Dan Asam Lemak Bebas Pada Crude Palm Olein. Karyailmiah [Program Studi Diploma] Kimia Departemenkimia :Universitas Sumatera Utara.
Haas,
M. J. Scott, K.M., Alleman.T.L. dan Mc Cormick, R.L.2001. (1207-12).
Engine Performance of Biodesel Fuel Prepared from Soybean Soapstock: A. High
Quality Renewable Fuel Produced from a waste Feedstock. Energy and Fuels, 15. https://doi.org/10.1021/ef010051x.
Knothe, G., and Gerpen, J. V. danKrahl, J. (editor). (2005). The biodesel handbook. AOCS press, Champaigh. IL. https://doi.org/10.1201/9781439822357.
Mardina, P., Faradina, E., and Setiyawati, N. (2012). Penurunan Angka Asampada Used Cooking Oil. Jurnal Kimia, 6(2).
Purwaningrum, S. D., and Sukaryo, S. (2018). Uji
Karakteristik Biodiesel Berbahan Dasar Limbah Jeroan Ikan Diproses Menggunakan
Mikro Gelombang. METANA, 14(2), 37-42. https://doi.org/10.14710/metana.v14i2.20333.
Sangha, M. K., Gupta, P. K., Thapar, V. K., and Verma. (2005). Storage Studies on Plants Oil and Their Methyls Esters. College of Agricultural Engineering, Punyab Agricultural University.
Setyawati, E., and Edwar, F. (2012). Teknologi Pengolahan Biodiesel Dariminyak Goreng Bekas Dengan Teknik Mikrofiltrasi Dantransesterifikasi Sebagai Alternatif Bahan Bakar Mesin Diesel, Jurnalriset Industri, 6(2), 117-127.
Soerawidjaja, T. H. (2003). Standar Tentatif Biodiesel Indonesia Dan Metode-Metode Pengujiannya, Disampaikan Dalam Diskusi Forum Biodiesel Indonesia, Bandung, 11 Desember, 2003.
Traction Energy Asia. (2019). Pemanfaatan Used Cooking Oil Untuk Produksi Biodiesel Dan Pengentasan Kemiskinan Di Indonesia. TNP2K.
Wahyuni, S., Kadarwati, S., and Latifah. (2011). Sintesis Biodiesel Dariused Cooking Oil Sebagai Sumber Eenergi Aalternatif Solar. Jurnal Sain Danteknologi, 9(1), 51-62.
Wahyuni, S., Kadarwati, S., and Latifah. (2020). Pedoman dan penyimpanan biodiesel and B30. [Kementerian Energi dan Sumber Daya Mineral].
|
|
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© IJETMR 2014-2023. All Rights Reserved.


