|
|
|
|
A REVIEW ON CARBON/GRAPHENE QUANTUM DOTS AND THEIR APPLICTAIONS IN ANODE OF LITHIUM-ION BATTERIES
Iti Diwan 1![]()
![]() ,
Purnima Swarup Khare
2
,
Purnima Swarup Khare
2
1 Research Scholar, Department of Physics, Rajiv Gandhi Technical University, Bhopal, Madhya Pradesh, India
2 Professor and Director of
School of Nanotechnology, RGPV, Bhopal, Madhya Pradesh, India
|
|
ABSTRACT |
||
|
This article is all about a revolutionary carbon nanomaterial Carbon/Graphene quantum dot “(C/GQDs)". It is known as the world's strongest, lightest, thinnest, and hardest material, with essentially endless sources due to its composition of carbon, which is the fourth most abundant element in the universe. CQDs are carbon nanoparticles that are smaller than 10 nm. Strong and controllable fluorescence emission, structural and chemical stability, wide surface area, electrical conductivity, and low toxicity are just a few of the characteristics of these 0- dimensional QDs. This can be used in a variety of ways. In this paper, we'll discuss about their application in Lithium-ion batteries. These batteries are particularly promising energy storage devices because of their high capacity, fast charge-discharge rates, light weight, and great stability. These rechargeable batteries have proven to be a rising star, with plenty of opportunity to grow in order to meet future energy demands. This study will provide an overview of carbon quantum dots as an anode for Li-ion batteries, as well as the advantages of carbonic anodes. It also explains why carbon quantum dots and their composites are the best anode materials for lithium-ion batteries. We intend to offer a brief overview of several carbon anodes, as well as a thorough examination of various anodic materials that are now accessible. |
|||
|
Received 01 October 2022 Accepted 02 November 2022 Published 23 November 2022 Corresponding Author Iti Diwan, itidiwan9@gmail.com
DOI 10.29121/ijetmr.v9.i11.2022.1247 Funding: This Research
did not receive any specific grant from funding agencies in the public,
commercial or not for profit sector. Copyright: © 2022 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Carbon Based Nano-Composites, Carbon Quantum Dots, Carbon Nanotubes,
Electrochemical Performance, Graphene Quantum Dots, Lithium-Ion Battery ABBREVIATIONS
AND NOMENCLATURE Carbon Quantum Dots = CQDs, Graphene Quantum
Dots = GQDs, Lithium-ion Battery = LIBs, Sodium ion battery = SIBs, Carbon
Nano-tubes = CNTs, Reduced Graphene Oxide = rGO,
Transition metal oxides = TMOs, Transition metal carbonates = TMCs |
|||
1. INTRODUCTION
This paper is on a miraculous carbon nano material “Carbon/graphene quantum dots”. It is known as the strongest, lightest, thinnest, and hardest material on the planet and its sources are virtually limitless since it is made up of carbon, which is abundantly present in the nature. CQDs are small carbon nanoparticles with size of less than 10 nm Xu et al. (2004). These 0-dimensional CQDs have tremendous properties as- strong and tunable fluorescence emission, structural and chemical stability, large surface area, electrical conductivity, and low toxicity. This offers a wide range of applications in Photo-luminescence Li et al. (2011), Photo & Electro catalysis Zhuo et al. (2012), Tang et al. (2014), bio-imaging/sensing Li et al. (2017), Huang et al.(2014), Nano-medicine Zheng et al. (2015), Chemical sensing J. Wang (2017), Photovoltaics’ (solar cell) & Light Emitting Diode (LED) Gao et al.(2019), Yang et al. (2020) and super capacitor electrodes Sharkawy et al. (2020). Unfortunately, the study in the field of energy storage technology is still rare.
Li-ion batteries are a very promising energy storage device because of their capacity, charging speed, light weight, battery life, and stability; their remarkable electrochemical performance makes them commercially viable. Their superior features have had a direct impact on numerous electronic/power industries. As these batteries are the primary source of power for a wide range of applications in electric vehicles, they also dominate the market for portable devices such as laptops and mobile phones. commercially, Graphite is utilized as an anode material in industry. However, while graphite is readily available at a low cost and exhibits excellent structural stability, it has a limited theoretical capacity (372mAh/g) and poor rate performance Yoshino (2012) , which provoked researchers to seek out new materials as anodes with highly porous structures, large active surface areas, and good electric conductivity for improved Battery performance. CQD based composite can possess phenomenal electrochemical properties. In present paper, we review several CQD based Nano-composites as an anode for Li-ion battery. Preparing such material contains a lot of opportunities in research.
Figure 1
|
Figure 1 Illustrates Applications
of CQDs/GQDs. |
2. BACKGROUND; INTRODUCTION OF CARBON-BASED ANODE IN LIB
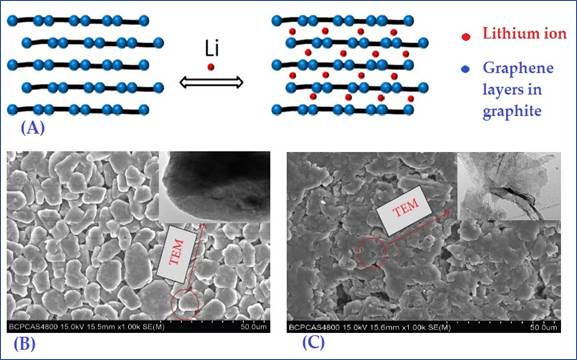
The development of a safe and stable anode had already made significant progress. Several substantial efforts to produce efficient anodes have been conducted in the previous decade. Carbonaceous materials and composites, transition metal oxides/carbonates/sulfides are a few of them. Because of their great stability, spectacular porous morphology, good electrical conductivity, and ease of ion transportation, carbon-based anodes were rated one of the most promising alternatives among them. Akira Yoshino was the first who introduced carbonaceous material in Li-ion battery. He invented the first rechargeable (secondary) battery in 1983, using Polyacetylene (C6H6) as the negative electrode and Lithium Cobalt Oxide (LiCo2) as the positive electrode Yoshino (2012). However, polyacetylene's poor density and limited chemical stability failed in this case. After that Yoshino was looking for a novel carbonaceous anode material; graphite with LiCo2 positive electrode. Since hybridization state of graphite is sp2 and hybridized graphene layers are linked by weak Van Der Waals forces of the delocalized electron orbitals in graphite. This weak intercalation between layers contributes to the easy intercalation of lithium ions into graphite, Figure 2 (A) illustrates lithium intercalation and deintercalation between layered structured graphite. Zhang et al. (2021), it has a high lithium intercalation capacity (372mAh/g), stability, and electrical conductivity Which makes it a suitable choice for an anode Xu et al. (2018). However, Heavy amount of high-quality graphite is used for making anodes which is most important component and plays crucial role in the performance of these batteries. This leads to the complicated costly manufacturing processes and batteries produced are heavy weight suffers significant shortcomings. Further the capacity and energy density of graphite anodes is low along with having concerning safety issues. Also, graphite has a tendency to decompose with liquid electrolyte during charging. Graphite anode's mechanical failure was caused by cracks over grain boundaries that were caused by the initiated volume change during repeated charging and discharging. These cracks frequently propagated over cycles. Presence of these cracks and fractures on the anode material was confirmed by SEM and TEM results in Figure 2 (B, C) Yoshino et al. (1985) , Lin et al. (2016).
Figure 2
|
Figure
2 (A) The Schematic
of Lithium Intercalation and Deintercalation Between Graphene Layers in
Graphite. [Reprinted with the Permission from the Publisher,
Copyright (2019) @RSC] Yoshino et
al. (1985). SEM Images of (B) Fresh Graphite-Based
Anode Before Cycling Tests and (C) Degraded Graphite-Based Anode After 500 Cycles.
[Reprinted with the Permission from the Publisher, Copyright © 2016
Cheng Lin et al. open access (HINDAWI), Creative Commons Attribution License] Lin
et al. (2016). |
Apart from these flaws, many other LIB electrode
materials suffer from rapid capacity fading and poor rate performance during
the charge-discharge process, which is caused by self-aggregation, uncontrolled
volume expansion in the electrode material, dissolution, the formation of a
solid electrolyte interface layer over the electrode, and a rapid increase in
charge-transfer resistance during cycles. Low Coulombic efficiency, electrolyte
depletion, and safety hazards are all prevalent problems with LIB electrode
materials. These issues in LIB electrode materials have recently been solved by
combining CQDs with LIB electrode materials and altering the surface states and
internal structures of the electrode materials to improve LIBs next-generation
efficiency Akash et al. (2021).
However, in the field of rechargeable energy
storage devices, CQD based anode was deemed as the most promising
candidate. With its incredible strength, high stability, brilliant porous
morphology, excellent electronic conductivity, and easy transportation of ions,
CQDs has the potential to revolutionize the world of rechargeable technology.
CQDs may even replace rechargeable electronic mainstay graphite/silicon in many
applications, as its properties surpass the capabilities of graphite/silicon in
many instances.
There is an urgent need to pay more attention in this field for more progress as- more knowledge about CQD materials and their composites is required, aiming to design more reliable and durable anode architecture with excellent capacity and stability. There is a need of more customized, cost effective, environment friendly and safe synthesis mechanism for the wide range production of highly efficient electrodes for commercially viable batteries.
3. CQDS/GQDS BASED COMPOSITES IN LIBS
3.1. INDIVIDUAL CQD/GQD STRUCTURE
Carbon quantum dots (CQDs) are nanoscale carbon particles having a diameter of less than 10 nanometers. X.U. et al. was the first who found these 0-dimensional CQDs accidentally in 2004 while purifying single walled carbon Nanotubes Xu et al. (2004). Similarly, Andre Geim and Konstantin Novoselov developed graphene, one of the most widely utilized allotropes of carbon, and were awarded the "Nobel Prize in Physics in 2010" for "Ground breaking experiments regarding the 2-dimensional material Graphene" Geim and Novoselov (2010). Graphene is a two-dimensional planar sheet of carbon atoms that is one atom thick. It has many structural and fundamental properties similar to carbon nanotubes Wang (2016). The features of these nano-sized carbonic dots are incredible, including - strong and tunable fluorescence emission, structural and chemical stability, huge active surface area and electrical conductivity, low toxicity and good conductivity, strong intercalation ability, low density, and outstanding mechanical and electrochemical capabilities are just a few of the many characteristics of these CQDs/GQDs. There applicability has already been witnessed in Photo-luminescence, Photo& Electro catalysis, bio-imaging, chemical sensing, solar cell, Light Emitting Diode (LED) and super capacitor electrodes. There are some reports on these QDs indicating their applicability in LIBs. The individual Graphene Nano Sheets has the ability to trap ions on both sides of each graphene sheet due to its stacked multilayered structure. Such type of report was firstly presented by Peng Guo et al in 2009. Graphene nano-sheets (GNS) were synthesized from artificial graphite by oxidation and ultrasonic treatment. Such prepared GNS presents Variety of voids and cavity which provides an excellent initial charge and discharge capacity of 1233 and 672mAh/g at 0.2 mA/cm2 rate. Good cycle performance and high-rate charge/discharge properties. But a huge loss of capacity was induced during the upcoming cycles Guo et al. (2009). It is suggested that large irreversible capacity of GNSs can be decreased by the surface modification through various methods. This kind of modification was performed by Tian L et al over GNS through chemical treatment in 2011. As a result, these GNS structure demonstrated great initial discharge and charge capacity of 1481.5 and 601mAh/g at 100mA/g current rate Guo et al. (2009).
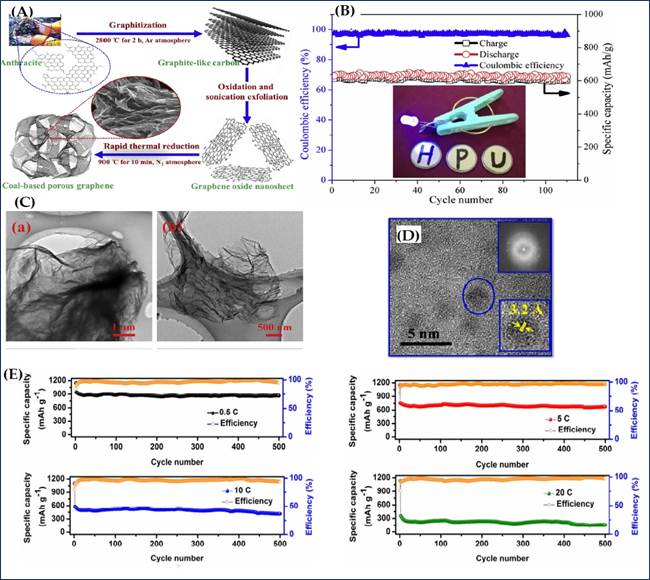
To achieve enhancement in rate performance, a coal based porous graphene structure (CPG) was developed in 2018 by Baolin Xing et al. They used an inexpensive graphitization, liquid oxidation and thermal reduction synthesis routes, the schematic is represented in Figure 3(A). their morphological studies revealed that CPG displays an extremely organized compact texture and layered stacking structure. Their continuous thin layered structure in large-scale with abundant wrinkles is confirmed via TEM micrographs which provides sufficient active sites for Li-storage Figure 3 (C)]. CPG demonstrated very high initial capacities and tremendous reversible capacity of 601mAh/g which maintained till 110 cycles, The retention rate of reversible capacity for CPG is up to 98.0% after 110 cycles, suggesting the superior cycling performance and fascinating cycling stability, denoted in Figure 3 (B). Moreover, a stable Coulombic efficiency close to 100% is also observed for CPG electrode during the entire cycling performance measurement, which further indicates the excellent long-term electrochemical stability Xing et al. (2019).
Figure 3
|
Figure
3(A) Schematic Illustration of the
Procedure for Preparation of CPG. (B)
Cycling Performance Along with Coulombic Efficiency at the Current Density of
1 C (Inset: Photograph Demonstration a 1.5 V Blue Light Emitting Diode
Powered by a Single Fabricated LIB Based on CPG as an Anode Material). (C)
TEM Micrographs of CPG: (a-b) TEM; Reprinted with Permission
from the Publisher, Copyright (2019) @ Elsevier. Xing et al. (2019). (D) TEM Images
of CQDs; Lower Inset Shows a HRTEM Image of an Individual CQD Displaying the Typical
Lattice Constant; Upper Inset Shows a SAED Pattern. (E) Cyclability with the Corresponding
CEs of the CQD Electrode at 0.5, 5, 10, and 20 C for 500 Cycles each. Reprinted
with Permission from the Publisher, Copyright (2018) @ Elsevier. Javed et al. (2018). |
A most advanced and unique CQD fabrication technique from
D- (+) glucose via chemical oxidation was introduced in the same year by M. Javed and fellows. Their TEM analysis presented in Figure 3 (D). Such prepared samples exhibited very high discharge and
charge capacities, superior rate performance and stabilized reversible
capacity, even after 500 continuous charge and discharge cycles, these CQD
electrode still delivered the high reversible-capacity values of 864.9, 674.0,
415.8, and 153.7mAh/ g at
0.5, 5, 10, and 20 C rates, respectively. The capacity-retention values of
91.6, 89.4, 71.3, and 42.8% were measured for 0.5, 5, 10, and 20 C rates,
respectively, see Figure 3 (E),
Javed et al. (2018). previous findings demonstrated the
potential of these readily available and environmentally friendly carbon and
graphene nano dots for electrochemical applications in the production
of LIBs.
3.2. CQD/GQD AND HETEROATOMS
However, there are various key advantages of G/CQDs anodes
as- structural/chemical stability (provide stability to the electrode and
hindered their decompositions with electrolytes), huge surface area and layered
structure (enlarged the ion accessible interface area, boost the
ion-transportation process with rich active sites in electrode), low toxicity
(provides easy and environment friendly synthesis approach for electrode),
small size (reduced the diffusion path between electrolytes and electrodes and increases
intercalation of ions), outstanding electrochemical capabilities Song
et al. (2020). CQDs still
possess large amounts of nonessential oxygen-containing groups at the surface,
which can prohibit Li+ accessibility and decrease electrical
conductivity. ![]() for dealing with these limitations CQDs have been widely studied as
composite agents and not as independent active materials. Most recently,
K H Kim and H J Ahn propose surface functional
group-tailored boron and nitrogen co-doped carbon quantum dots (BN-CQDs) in
2022. This type of electrode demonstrated extraordinary electrochemical
performance, including exceptional rapid energy storage capability (130.4mAh/ g at 3000 mA/ g with capacity
retention of 88% up to 1000 cycles). This is attributed to the
boron and nitrogen co-doped structure's increased electrical conductivity and
high Li+ acceptance, which enabled the creation of C═O surface functional
groups due to the boron dopant Kim
and Ahn (2022).
for dealing with these limitations CQDs have been widely studied as
composite agents and not as independent active materials. Most recently,
K H Kim and H J Ahn propose surface functional
group-tailored boron and nitrogen co-doped carbon quantum dots (BN-CQDs) in
2022. This type of electrode demonstrated extraordinary electrochemical
performance, including exceptional rapid energy storage capability (130.4mAh/ g at 3000 mA/ g with capacity
retention of 88% up to 1000 cycles). This is attributed to the
boron and nitrogen co-doped structure's increased electrical conductivity and
high Li+ acceptance, which enabled the creation of C═O surface functional
groups due to the boron dopant Kim
and Ahn (2022).
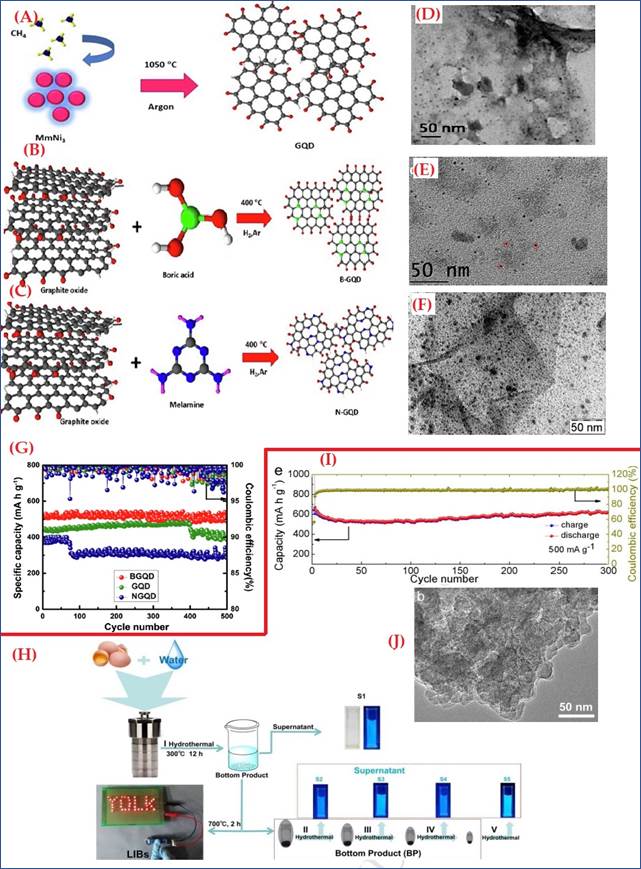
According to a published report. In 2020 undoped GQDs and dopped (boron & nitrogen) GQDs were synthesized via CVD technique, schematic is represented in Figure 4(A-C). Morphological study of which demonstrated in figure 4(D-F), TEM images verified that GQDs (Figure 4D) are uniformly distributed throughout and have a high surface area, where a significant amount of lithium is used to form the SEI layer. The magnified TEM image of B-GQD (Figure 4E) revealed that, boron doped quantum dots are uniformly distributed and nearly the same size throughout the entire region. Numerous theoretical studies have demonstrated that adding boron to graphene results in an electron-deficient system and increases the sites' ability to store Li+ ions. Additionally, boron is an effective material for lithium storage because one boron atom doped into the carbon matrix can hold six Li+ ions. The TEM image of an N-GQD (Figure 4F) clearly indicates that the N-GQDs are uniformly distributed and highly crystalline in nature. The nitrogen doping sites in N-GQD, results defects in the graphene structure and help Li+ ion storage properties, which are responsible for the enhanced Li ion storage capacity of N-GQD. The electrochemical studies of B-GQD, N-GQD and GQD demonstrated high reversible capacities of 660, 521, 400mAh/g respectively, at current rate of 50mA/g, The long-term cyclic ability was also maintained at 200mAh/g even after 500 cycles with coulombic efficiency of almost 100% Saroja et al. (2020). Almost 2 years ago, S. Wang et al prepared nitrogen doped CQD from egg yolk as both a carbon and nitrogen source. The hydrothermal synthesis technique for N-CDs and LIBs is shown in, Figure 4 (G). as we have discussed earlier that Nitrogen is a well-known dopant, and proper content of nitrogen can surely enhance Electro-chemical properties of any carbon matrix. TEM image in Figure 4(H) confirmed that its layered structure, high surface area and small diffusion path provided high rate and cycling performance and better ionic transportation Wang et al. (2018). such prepared N-CQDs demonstrated high rate and cyclic stability. In Figure 4 (I), the capacity increases gradually and keeps stable at 601.0mAh/g even after 300cycles with almost 100%CE.
In 2020, Another approach was introduced where, D.Y. Shin and colleagues used electrochemical etching and spray coating techniques for F-CQD (hydrothermal and post-calcinations) directly onto Cu foil, [Figure 5(a-d)]. According to Strong Metal-Support Interaction (SMSI), F-CQD and Cu foil mutually demonstrated very strong interaction, which inherently increases the bond between F-CQD and Cu foil. Due to its unique quantum effect, C–F bonds and high step coverage the F-CQD interfacial layer not only demonstrated high electrical conductivity and excellent chemical stability but also can effectively prevented the corrosion of the Cu foil by the decomposition of the electrolyte. As a result, including the F-CQD interfacial layer on the etched Cu foil was a successful technique for increasing charge transfer and preventing electrochemical degradation. Even after 100 cycles at 100mA/g current density, F-CQD @ etched-Cu foil had a specific capacity of 297.3mAh/g with 94.3% capacity retention, which was clearly better than bare Cu (specific capacity-251.4mAh/g with capacity retention-80.9%) and etched Cu (specific capacity-279mAh/g with capacity retention-89.2%) see the cyclic performance in figure 5(e) These samples have excellent area coverage over Cu foil, resulting in increased ionic/electric conductivity and a simple ion transport channel.
In case of Graphene
anodes, they have a number of advantages, but they
also have certain drawbacks, such as the integration of agglomerates during
cycles, which limits their large-scale production of Graphene composites. This
can be solved by using composites of graphene rather than pure graphene. Jiantie Xu and colleagues introduced nitrogen doping on
holey graphene in 2015. As a result, at 0.1A/g current rate, N-hG produced initial discharge and charge capacities of
3056.1 and 989.5mah/g, respectively. These findings suggest that N-hG has high packing densities and a good ion insertion and
extraction tendency, making it an effective anode. Its discharge capacity was still maintained
to 553.5mAh/g after 6000 cycles at a very high current rate of 5A/g, whereas
pure graphene had only 198.1mAh/g Xu et al. (2015). In comparison to pristine graphene, their
findings indicated that Nitrogen doping on holey graphene provides much greater
initial capacities and stabilities.
Figure 4
|
Figure
4 Schematic of Synthesis of (A)
GQD, (B) B-GQD and (C) N-GQD. TEM Images
of (D) GQD, (E) B-GQD, (F) N-GQD. (G) Cyclic Stability at 200 mA g−1 for
B-GQD, N-GQD and GQD LIB Anodes. Reprinted with Permission from the Publisher,
Copyright © 2019 Elsevier B.V. Saroja
et al. (2020). (H) The Synthesis
Process of the N-CDs and Anode Materials for LIBs. (I) Cycling Performance of
the B1 at a Current Density of 500 mA/g. (J) TEM Image of B1. Reprinted with Permission from the Publisher,
Copyright (2018) @ Elsevier. Wang et al.
(2018) |
Figure 5
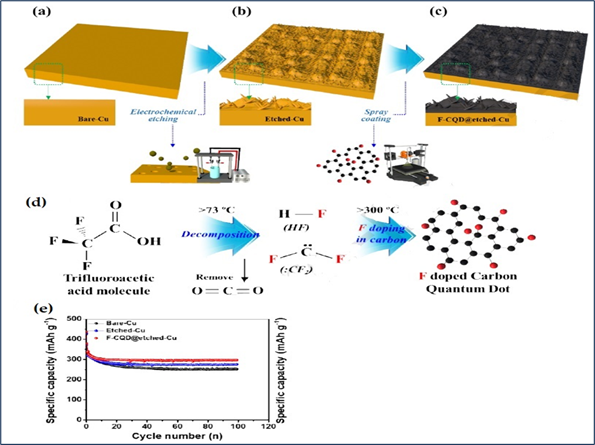
|
Figure 5 (a) Schematic
Illustration of Electrochemical Etching and Direct Spray Coating of a Bare Cu
Foil, (b) Electrochemically Etched Cu Foil, and (c) F-CQD Coated Etched Cu Foil
to Fabricate the F-CQD Interfacial Layer on Stockade-like Etched Cu Foil. (d)
Schematic Illustration of F Doping Process into Carbon Quantum dot.
(e)Cycling Test of all Sample Electrodes at a Current Density of 100 mA/g up
to 100 cycles. Reprinted with Permission from the Publisher, Copyright
(2020) @ Elsevier. Shin et al. (2020) |
3.3. CQD/GQD AND TRANSITIONAL METAL OXIDES/CARBONATES
Making composites of CQDs with metal oxides improves the material's stability and electrochemical performance. Because of the extraordinary advantageous features of CQDs this material is being undertaken all over the world. CQDs and metal oxides have both made significant contributions to energy storage applications. However, their composite illuminate's high reversible capacity, but caused agglomerates, volume volatility, and poor electrical conductivity necessitate various changes in sample manufacturing and structure Zhang et al. (2015), Zhu et al. (2013), Huang et al. (2016). This type of modification is practiced by M. Jing et al., who; submitted the first report in this respect in 2015. They used a composite of manganese oxides (Mn3O4) and CQDs. Manganese oxides have already been used as anodes Tarascon et al. (2000), but their low conductivity and induced pulverization have limited their commercial acceptability. These shortcomings could be remedied by using a carbon matrix in their composites. An electro-chemical technique was used to create a CQD coated Mn3O4 sample. CQD coating can reduce volume variation and improve the ion transportation, resulting in a significant improvement in cycling stability and rate performance, and capacity was maintained to 791mAh/g even after 100 cycles Jing et al. (2015), which was undoubtedly better than bare Mn3O4 (114mAh/g after 100 cycles). In 2021, a research team used the hydrothermal technique to create graphene quantum dots (GQDs)-coated hierarchical nanoflake-based CuO microspheres (H-CuO) composite on Cu foam. Figure 6 (A) shows the schematic representation. In addition to lowering charge transfer resistance, the GQD coating also protects the electrode from degradation and agglomeration. FESEM reports of pure H-CuO and GQDs/H-CuO composite films clearly show the formation of microspheres packed with nanoflakes. Its morphological studies are shown in figure 6(B-C). These prepared samples showed impressive reversible capacity of 609mAh/g (pure H-CuO: 61mAh/g) even after 200 cycles at 0.2A/g with improved initial coulombic efficiency of 88.2% (pure H-CuO: 75.2%). [ figure 6(D)]. The superior electrochemical properties of the GQDs/ H–CuO composite anode are attributed to graphene networks, which provided a high specific surface area and successfully protected the anodic active material from developing an unstable solid electrolyte interface layer Kim et al. (2021). According to previous reports, the majority of the metal oxides experienced volume variation, which caused severe or permanent damage to the battery's conductive network. In order to avoid such situations self-healing materials and CQD composites have captured the attention of scientists. In 2020, J. Gua and colleagues used a simple microwave and chemical treatment to create a hollow Ga2O3@N-CQD composite as a self-healing anode, schematic is presented in Figure 6(F). Gallium content gave it the ability to self-heal (due to its lower toxicity and lower melting point (29.80C)). The diffusion path between electrode and electrolyte was reduced by the hollow structure. The structural stability and electrical conductivity were provided by the N doped CQD layer. Their HRTEM images in figure 6(G-H) also confirmed these facts. The well-designed structure of the H-Ga2O3@N-CQDs can offer an excellent foundation for its superior electrochemical performance. It provided a high discharge and charge capacity as well as good sample control. After 1000 cycles at 2A/g rate, an excellent reversible capacity of 410mAh/g was achieved with a CR of 100%. Their comparative cycling performance is presented in figure 6(I) Guo et al. (2020), which was undoubtedly far superior to any other gallium-based nanostructures.
Z. Deng and T. Liu demonstrated a nanostructure of Bi2O3-rGO
in late 2017. Bismuth oxide nanoparticles of single crystal type were evenly
distributed throughout the surface of reduced graphene oxide sheets as shown in
figure 7 (E). This generated samples
demonstrated superior reversible capacity of 347mAh/g at 1C rate after 100
cycles with 79% CR, which was unquestionably superior than
bare -Bi2O3 (169mAh/g with 43%-CR) Their comparative rate
performance curves are shown in figure: 7(A)
Deng
et al. (2017). Another study found that
Bi2O3 and CQD composites provided excellent stability and
prevented electrode cracking. A. Prasath et al created a CQD-Bi2O3
composite using a hydrothermal technique in 2019. Its unique construction
guaranteed improved ion transport and a very high discharge capacity of
300mAh/g after 30 cycles at 100% CR, shown in figure:
7(B) Prasath
et al. (2019). Z. Xu and his
colleagues investigated molybdenum trioxide and CQD composites the same year
Individually, MoO3 has a specific capacity of 1117mAh/g, which is
adequate; however, its poor electrical conductivity and large volume
change limit its viability. To avoid these difficulties, a hydrothermal MoO3/CQD
composite was created. Such doped active and motile CDs induce MoO3
to form a rice Krispie’s sphere-like structure shown in figure 7(F), which provides an excellent interface for
intercalations between electrodes and electrolytes, resulting in desired
reversible composite reactions. These composites provided excellent rate
performance as capacity remained stable at up to 890mAh/g at 0.1A/g rates with
71.2% CE Even after 100 cycles. Their exceptional rate performance is
shown in figure 7 (C) Xu
et al. (2019).
Transition metal carbonates (MnCO3, CoCO3,
ZnCO3, etc.) have also been studied as potential electrode
materials. B. Jiang et al developed a FeCO3 and N-doped CQDs anode (FeCO3/N-CQD)
in 2020 using a one-step hydrothermal synthesis technique, see figure 7(G). The CQD content stabilize the control
over volume expansion. Their distinctive hierarchical porous structure and
large surface area indicated improved electrochemical reactions. Like even
after 460 cycles, its reversible capacity reached 872mAh/g at 200mA/g current
rates, rate performance is shown in figure 7(D) Jiang et al. (2020).
We have discussed various strategies to enhance the current and next generation
systems, where a sophisticated approach will be needed to unlock higher energy
density while also maintaining lifetime and safety.
Figure 6
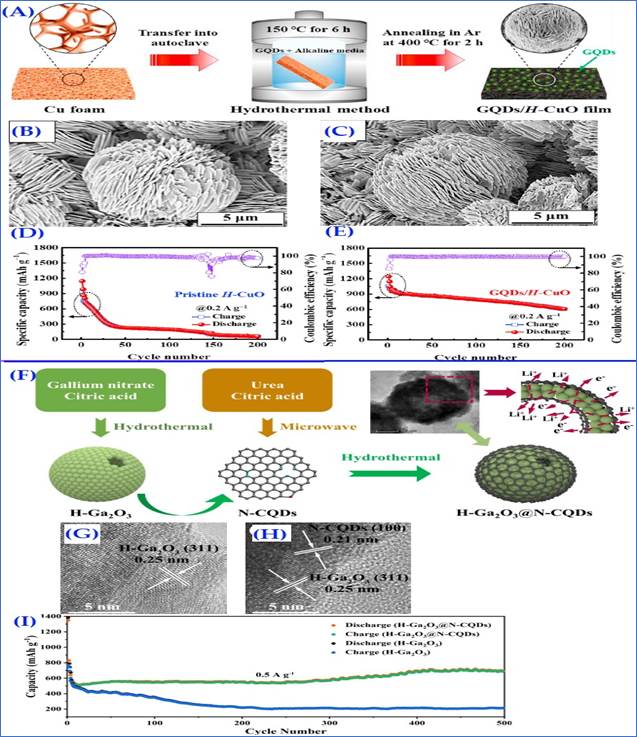
|
Figure 6 (A)
Schematic Illustration of Process for Fabricating GQDs/H–CuO
Composite Film Electrode Via Hydrothermal Method, (B-C) FE-SEM Images of Pristine
H–CuO and GQDs/H–CuO,
(D-E) Cycling Performances and Coulombic Efficiencies of Pristine H–CuO and GQDs/H–CuO Composite Anodes
at Current Densities of 0.2 A/g. [ Reprinted with the Permission from
the Publisher, CC BY-NC-ND license ©2021 Elsevier Kim
et al. (2021); (F) The Synthesis
Schematic Illustration of the H-Ga2O3@N-CQDs nanospheres, (G-H) HRTEM Images
of the H-Ga2O3, and H-Ga2O3@N-CQDs, (I) Cycling Performances of the H-Ga2O3
and the HGa2O3@N-CQDs Electrodes at 0.5 A/ g, the First Three Cycles are
under 0.1 A/ g [Reprinted with Permission from the Publisher, Copyright
(2020) @ ACS] Guo
et al. (2020). |
Figure 7
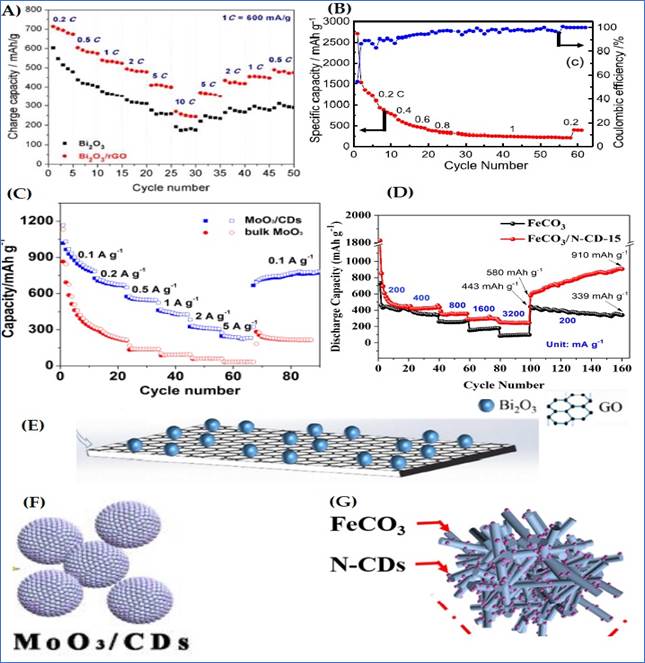
|
Figure 7 (A)
High-Rate Performances at Different C Rates Bi2O3 and Bi2O3@rGO
nanocomposite; [Reprinted with Permission from the Publisher,
Copyright (2017) @ ACS open access] Deng et al. (2017).
(B) Cycle Life Data and Coulombic Efficiency Obtained at Different C Rates [Reprinted
with Permission from the Publisher, Copyright (2019) @ ACS open access] Prasath et al. (2019).
(C) Rate Performance of MoO3/CDs and bulk MoO3 [ Reprinted with Permission
from the Publisher, Copyright (2019) @ WILEY-VCH]
Xu et
al. (2019)
(D) Rate Capacities of the Pristine FeCO3 and FeCO3/N-CD anodes at different Current
Densities, [Reprinted with Permission from the Publisher, Copyright (2020) @
Elsevier] Jiang et al. (2020). (E-G) Schematics of
Bi2O3@rGO, MoO3/CDs and FeCO3/N-CD nano-composites. |
|
Table 1 Illustrates Some CQDs/GQDs based NCs- their Synthesis
Routes, Advantages, and Reversible Capacity
Per Cycles at Specific Current Density |
||||||
|
S.N. |
Composite |
Synthesis Technique |
Advantage |
Efficiency |
Year/ ref |
|
|
1 |
(Co, Mn)3O4
/ rGO |
Hydrothermal |
Due to the
synergetic effects of Co, Mn, and graphene embedded
sample |
Reversible
capacity of 750mAh/g was achieved at 1A/g, even after 140cycles |
2016/ Li et al. (2016) |
|
|
2 |
SnO2/rGO |
Hydrothermal |
By virtue of
stable nature and special architecture of 3D-rGO sample. This composition
occupied better results compared to individual SnO2 and rGO. |
Reversible
capacity of 573mAh/g was achieved at 1A/g rate even after 420 cycles. |
2019/ Tan et al. (2019) |
|
|
3 |
NiO@Co3O4@GQDs |
Hydrothermal
and solvothermal |
Its
bimetallic hollow spherical structure provides large pore volume and GQD
provides more surface area |
Reversible
capacity of 803mAh/g was achieved at 1A/g rate even after 500 cycles. |
2019/ Yin et al. (2019) |
|
|
4 |
Yolk-Shell-Co3O4/CQD |
In-situ technique |
The yolk–shell structure with hollow cavity and
interior porosity offers numerous active sites and the addition of a
CQDs-decorated layer increased the ionic/electric conductivity and structural
stability. |
Reversible capacity of around 1027mAh/g at 0.1A/g. |
Zihao He et al.
(2022) |
|
3.4. CQDs/GQDs AND SILICON
One of the most essential ways for boosting charge storage
capacity of any material is to add silicon, which has the maximum possible
charge storage capacity of 3579 m Ah/g. Silicon anodes with ten times the
capacity of carbon anodes has gained a lot of research attention as a way to tackle the severe volumetric change problem that
plagues alloy anodes Qi
et al. (2017). Silicon is another extremely promising anode material as each Si
atom is able to capture around 4 Li atoms through a
conversion reaction in the lithiation process. This results in its highest
gravimetric and volumetric capacity among all the elements known and also is of low cost. The working potential of Si-based
anodes is also low which can avoid risks compared with graphite electrodes.
However, the high lithium storage capacity of Si anodes leads to a large volume
expansion, Wang
et al. (1986), Wen
and Huggins (1981), Kim
et al. (2004), Kierzek et al. (2015) causing huge volume
variation which is a significant disadvantage associated with the application
of Si. Low conductivity and the solid-state phase transition, which results in
massive irreversible capacity loss, are the other roadblocks. To address these
issues, nano structuring Si for use as an anode material has been proposed.
Another approach is to use carbon nanomaterials as additive materials for
Si-based composite electrodes because they are tunable,
have excellent electrical and mechanical properties, and are lightweight. Luo
et al. (2015) Si/C composite anodes
have been extensively researched in a variety of frame architectures. Silicon
has a high specific and volumetric capacity but suffers from significant volume
expansion and contraction, whereas the carbon matrix could accommodate the
volumetric fluctuations, preserve electrical stability, and structural
integrity while reducing battery capacity. As a result, the silicon content of
the Si/C composite has a significant impact on the attributes of rechargeable
batteries Bridel et al. (2010), Zhu
et al. (2015), Ren et al. (2015), Ren
et al. (2015).
In this perspective Yangzhi Bai et al. created a silicon/carbon composite in 2021. By using a spray drying technique that reduces the risk of silicon agglomeration and a double heat treatment that can prevent volume expansion, and provide uniform and dense coating of carbon over silicon composite. Systematic synthesis route is represented in figure 8(A). Their morphological studies confirmed that a double coating of nano-silicon and graphite by carbon layers is also very helpful in preventing direct contact between silicon/carbon and the electrolyte and preventing the rapid growth of the SEI membrane, see TEM results in figure 8(B). As a result, the silicon/carbon material's demonstrated initial charge and discharge specific capacity of- 936.4mAh/g and 1056.4mAh/g at 100mA/g rates. its initial coulombic efficiency is 88.6% and cyclic stability also increased, see in figure 8(C) Bai et al. (2021). Another spherical onion like silicon and carbon (Si/C) composite was developed by D. Wang et al in 2019 via one step simple direct injection pyrolysis technique, see in figure 8(D). TEM image in figure 8(E), illustrates the onion like coating structure and it can be found that the carbon-coating is ordered. This composite demonstrated exceptional Li-storage performance, with capacity as high as 1391mAh/g after 400 cycles at a current density of 0.2 A/g and rate capacity retention of 63.9% at 2 A/g to 200 mA/g, see in figure 8(F). Its superior electrochemical performance may be attributed to its unique onion-like structure, as well as its excellent control over ion and electron stability and transport Wang et al. (2019). The battery capacity would be increased by maximizing the amount of carbon used to relax strain produced during the lithiation/de-lithiation process, though stable cycles could be guaranteed through careful surface coating material selection and heat treatment with high security through appropriate research.
Figure 8
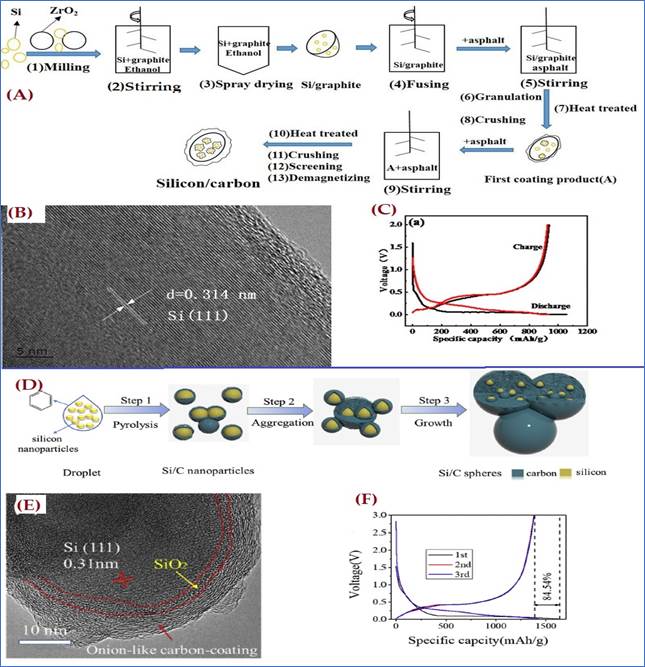
|
Figure 8 (A)
Synthesis Process of Silicon/Carbon Composite, (B) TEM Image of Silicon/Carbon
Composite, (C) Charge-Discharge Curves of Silicon/Carbon at a Current Density
of 100 mA/g. Reprinted with Permission from the Publisher, CC
BY 3.0 licence @ (2021) IOP Publishing Bai et al.
(2021). (D) Schematic Illustration of the Formation
of Onion-like Si/C Spheres, (E) HRTEM Images of Sample, (F) The Charge-Discharge
Curves of Si/C Composite. Reprinted with Permission from the Publisher, Copyright
(2019) @ Elsevier. Wang
et al. (2019) |
Figure 9
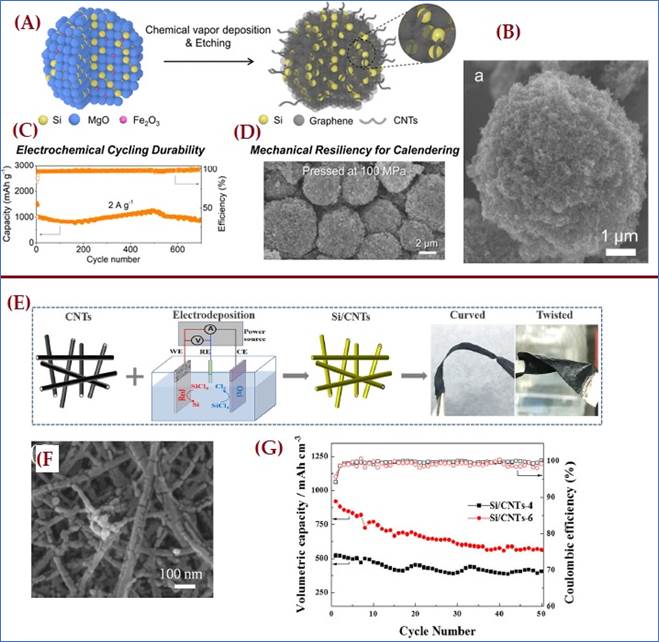
|
Figure 9 (A) A Synthetic
Schematic of in Situ Growth of Spheres of Graphene and Carbon Nanotubes
(CNTs) Around Silicon Particles. (B) and (D) SEM Image of an Individual
G-Si-CNTs Sphere with Different Magnifications. (C) Cycling Performance of
sample at 2A/g Current Density. Reprinted with the Permission from the Publisher
Copyright © 2022 American Chemical Society Xu et al.
(2022). (E) Schematic
of the Synthesis of the Si/CNTs and the Photographs of this Composite at Curved
and Twisted States. (F) Plan-view SEM Images of Si/CNT Composite (G) Cycling Performance
of these Two Electrodes at the Current Density of 800mA/g. Reprinted
with the Permission from the Publisher 2018© Frontiers in Chemistry Fu et al.
(2018). |
Most recently, in 2022, carbon nanotubes (CNTs) and graphene spheres were grown around silicon particles to create novel anode materials using a most promising chemical vapor deposition (CVD) technique, schematic is represented in figure 9(A). These composite materials can withstand the high pressure and induced stress produced during the charging and discharging of the electrodes because of their high mechanical resilience and electrical conductivity. The resulting electrodes have a sufficient volumetric capacity of 1006mAh/cm3, excellent cycling durability of 90% capacity retention at 2 A/g after 700 cycles and superior cycling performance, as shown in figure 9(C). SEM reports confirmed their standard morphology shown in figure 9(B, D) Xu et al. (2022). Few years ago, in 2018 Fu J et al also introduced; a paper like flexible Silicon/CNT composites via low-cost Electro-deposition technique, in which silicon layer was uniformly electro-deposited over the CNT substrate, their synthesis approach and morphological analysis SEM report are presented in figure 9 (E, F). This anode was very flexible and feasible for the development of flexible electronic devices such as thin and light weight Li-Ion Batteries Fu et al. (2018). Their exception cycling performance shown in figure 9 (G), and superior coulombic efficiency attributed to its higher tensile strength and high volumetric capacity. This paper like flexible Si/CNT composite was one of the most promising combinational candidates for LIB applications.
CVD, sol–gel, pyrolysis, mechanical milling, hydrothermal, and electro spinning processes are the most often used synthesis methods for silicon/carbon composite anode materials. Unfortunately, most standard synthesis techniques are low-yielding and unfriendly to the environment; as a result, it is necessary to develop scalable processes that will allow us to attain high productivity for the practical application of Si-based composite anodes Ren et al. (2016), Qi et al. (2017).
4. ADVANTAGES OF USING CQDs/GQDs AS
ANODE FOR LIBs
The basic principle of LIBs is that ions are stored in the anode terminal and then transported to the cathode terminal via electrolyte, schematic representation in figure 10(A). In lithium-ion batteries, the anode materials serve as the host, permitting reversible lithium-ion intercalation and deintercalation during charge and discharge cycles. Anode material is important in this case because the quality of the anode material has a direct impact on battery performance Nitta et al. (2015) . A suitable intercalation-based anode material must meet a number of general requirements, [figure 10(B-C) including
· It should demonstrate high reversible capacity, by other means the irreversible loss should be as low as possible.
· It should have a porous structure with a large active surface area which makes easy insertion and extraction path for ions and electrons.
· A high specific capacity, high coulombic efficiency, long durability, low toxicity, and a simple, low-cost synthesis approach.
· It should have quick lithium-ion diffusion into and out of the anode and small diffusion path.
· It should demonstrate high ionic, mechanical and electronic conductivity.
· It should have high volumetric stability by other means minimal structural changes during charge and discharge.
· It should have the ability to form and maintain stable SEI (Solid Electrolyte interface) layer upon cycling.
By virtue of
these requirements there is a genuine need for today's research to properly
select and control anode material. It would be foolish to assume that
"conventional" lithium-ion batteries are nearing the end of their
useful life. We conclude by quickly summarizing the areas where fundamental
scientific advancements will be required to make way for ground-breaking new
battery systems. Luminescent CQDs/GQDs are fascinating new researchers to the
world of nanomaterials, with variety of applications in energy fields. The
main synthesis methods and electrochemical properties of CQDs are discussed in
this paper, with an emphasis on their application in the energy field. Because
of their unique and intrinsic characteristics, CQDs have a
number of critical applications in the emerging field of energy storage
and conversion. The importance of using a larger capacity and green energy
conversion is becoming increasingly recognized among researchers and the
scientific community.
Figure 10
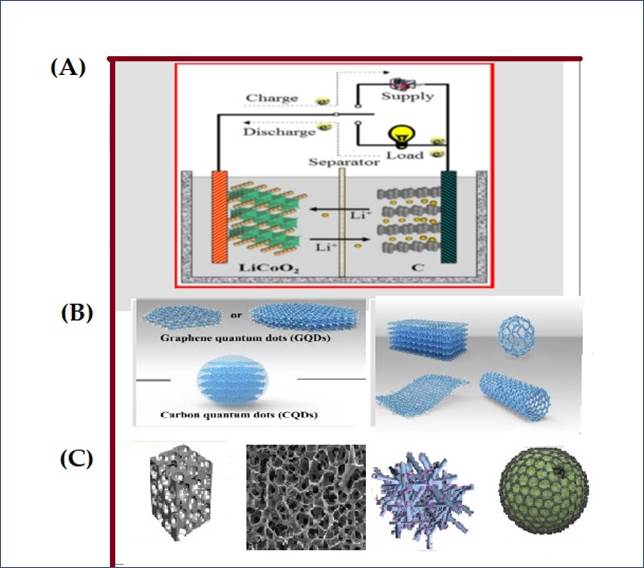
|
Figure 10 (A) Illustrates the Basic Principle and Operation of a
Li-ion Battery. Reprinted with Permission
from the Publisher, Copyright (2019) @ RSC.Qi et al. (2017). [B] Illustration of Carbon and Graphene Quantum Dots
Demonstrating their Layered and Porous Structures. [C] Highly Porous, Large Surface
Area Hierarchical Porous Carbon [Reprinted with the Permission from the
Publisher © 2020 Licensee MDPI (CC BY)] Zhao et al. (2020), Activated Porous Carbon [Reprinted with the Permission
from the Publisher © 2021 Licensee MDPI (CC BY)] Issatayev et al. (2022), FeCO3/N-CD [ Reprinted
with Permission from the Publisher, Copyright (2020) @ Elsevier] Jiang et al. (2020), HGa2O3@N-CQDs [Reprinted
with Permission from the Publisher, Copyright (2020) @ ACS] Guo et al.
(2020). |
CQDs/GQDs-modified nanomaterials have been the subject of a lot of research in recent years. Hydrothermal and microwave synthesis methods have the upper hand, Because of the ease with which the composition and morphology can be controlled. A large surface area and consistent particle size can be achieved using these methods. CQDs' electronic and chemical structures can be influenced by their size, shape, surface functional groups, making composites with electroactive materials and heteroatom doping. It's worth noting that increasing electrical conductivity through doping and modification improves their physiochemical properties. The CQD-modified electrodes perform admirably as anodes, reducing volume expansion, increasing ion diffusion rate, improving electronic double-layer kinetics, improving interfacial electrical conductivity and electrochemical performance, when used in LIBs and SIBs Todd et al. (2010). Therefore, large numbers of researches are now taking place with enhanced battery performance. Herein previous reports stated that 0-dimensional (CQDs, GQDs), 1D (CNTs, Nano-fibers, Nano-rods, Nano-wires) and 2D (graphene sheets, carbon sheets) have already justified themselves as powerful candidate for LIB application.
The additional coating of CQDs/GQDs on TMOs nano-particles can further improved the battery performance. Taking an example of Sn (semiconductor metal), SnO2 (transition metal oxide) and silicon all of them provided very high theoretical capacity as Sn (992mAh/g), SnO2 (782mAh/g) and Si (4200mAh/g) Zhang et al. (2015), Kamali and (2010), Liu et al. (2016). This is almost 2-10 times better than the commercially used graphite (372mAh/g). But rapid volume expansion during consecutive charging and discharging results unavoidable cracking and permanent damage of anode material, which introduced micro cracks or pulverization during transportation of Li-ions, their poor cyclic performance, unstable solid electrolyte interface and lower electrical conductivity are also the reasons behind the downfall of their performance. By introducing carbon matrix over these nano-particles can provide better stability and strength to the anode. R. P. Liu et al had demonstrated that carbon coating over Sn-SnO2 anode provided very high initial charge and discharge capacities of 878.7 and 1061.6mAh/g, respectively, with an initial CE of 82.3%, which is higher than any other reported results Zhang et al. (2020). Similarly, there are many other TMO (MnO2, MoO3, Fe2O3, Co2O3…) nanostructures which performed much better when embedded with carbon matrix. We have already discussed about some of them previously. In Si-C composites, silicon content provides extremely high capacity; on the other hand, carbon content accommodates the volumetric change, holds electrical stability, cyclic performance and maintained structural integrity Lu et al. (2017), Deng et al. (2009). Recently it is a trend to mix highly electro-conductive materials (TMOs, TMCs, alloys, silicon NPs) which gives rise in reversible capacities and cyclic performance of battery with highly stabilized and porous carbonaceous materials (CNTs, CQDs, Graphene, GQDs, CNWs, CNFs) which provides excellence in CE and great battery life.
5. Conclusion and future aspects
Despite recent progress in the development of CQDs and
their applications, the problem has not yet been resolved. When no size limit
is imposed on CQDs' growth, they expand in extremely wide ranges. Furthermore,
there are unknown properties in CQDs that have yet to be investigated, which
leaves their nanoscale chemical and physical nature undefined, confining their
application in energy storage devices. Another pressing issue confronting CQD
industrialization and synthesis that must be addressed is the rapid evolution
of their raw material sources. Apart
from that, there is a lack of consistency in the ability to reduce the
manufacturing cost of CQDs, which is obstructing their large-scale application
and commercialization. The ultimate goal is to combine
environmentally friendly, low-cost, low-toxicity, and efficient production
methods. There are still areas where more work can be done to improve the progress
made in this synthesis.
Despite the fact that CQDs have been shown to play a tremendous role in energy applications, a thorough understanding of the underlying mechanism and process, as well as important knowledge of electrochemical performance, remains a challenge. CQDs with high QYs are still rare and expensive. Future research should focus on improving the high QY, as well as chemical stability and photostability. Application-focused research should concentrate on improving CQD quality, selectivity, and reliability for energy-driven platforms at the same time, find out convenient set of electrodes that can compliments each other’s properties, right electrolyte combination is required to avoid damaging reactions associated with electrolyte and electrode interface. To better realize the potential of these increasingly important carbon materials, we anticipate the development of more cost-effective, simple, and revolutionary synthetic methods, as well as novel promising energy applications in the future. researching new anode materials and fabrication strategies for Li-ion batteries will undoubtedly have a bigger impact in the near future.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
The Authors gratefully acknowledge Dr. Purnima Swarup Khare, Professor and director of School of Nanotechnology Rajiv Gandhi Proudyogiki Vishwavidyalaya (RGPV) Bhopal, State Technological University, 462033, India for their Guidance. This Research did not receive any specific grant from funding agencies in the public, commercial or not for profit sector.
REFERENCES
A. D. W. Todd, P. P. Ferguson, M. D. Fleischauer and J. R. Dahn (2010). "Tin-Based Materials as Negative Electrodes For Li-Ion Batteries: Combinatorial Approaches and Mechanical Methods", International Journal of Energy Research, 34, 535-555. https://doi.org/10.1002/er.1669.
A. P. Vijaya Kumar Saroja, M. S. Garapati, R. Shyiamala Devi, M. Kamaraj and S. Ramaprabhu (2020)." Facile Synthesis Of Heteroatom Doped and Undoped Graphene Quantum Dots as Active Materials for Reversible Lithium And Sodium Ions Storage" Appl. Surf. Sci., 504, 144430. https://doi.org/10.1016/j.apsusc.2019.144430.
A. Prasath, M. Athika, E. Duraisamy, A.S. Sharma, V.S. Devi, and P. Elumalai (2019). "Carbon Quantum Dot-Anchored Bismuth Oxide Composites as Potential Electrode for Lithium-Ion Battery and Supercapacitor Applications", Journal of American Chemical Society, ACS Omega, 4, 4943−4954. https://doi.org/10.1021/acsomega.8b03490.
A. Yoshino, K. Sanechika and T. Nakajima (1985). "Secondary Battery", Japanese Patent, Assigned to Asahi Kasei, May.
A.Yoshino (2012). "The Birth of the Lithium-ion Battery", Article of Angewandte Chemie International Edition, 51. https://doi.org/10.1002/anie.201105006.
Akash S. Rasal, Sudesh Yadav, Anchal Yadav, Anil A. Kashale, Subrahmanya Thagare Manjunatha, Ali Altaee, and Jia-Yaw Chan(2021). "Carbon Quantum Dots for Energy Applications: A Review", ACS Appl. Nano Mater, 4, 6515−6541. https://doi.org/10.1021/acsanm.1c01372 .
Ali Reza Kamali and Derek J. Fray (2010)."Review on Carbon and Silicon Based Materials as Anode Materials for Lithium-Ion Batteries", Journal of New Materials for Electrochemical Systems, 13, 147-160.
Andre Geim, Konstantin Novoselov (2010). "The Nobel Prize in Physics", Nobel Prize.org., Nobel Media, Prize Announcement, AB2021.
B. Jiang, Y. Liang, X. Yu, G. Yuan, M. Zheng, Y. Xiao, H. Dong, Y. Liu, H. Hu (2020). "Facile Synthesis of Feco3/Nitrogen-Doped Carbon Dot Composites for Lithium-Ion Battery Anodes", Journal of Alloys and Compounds. https://doi.org/10.1016/j.jallcom.2020.155508.
B. Zhu, Y. Jin, Y. Tan, L. Zong, Y. Hu, L. Chen, Y. Chen, Q. Zhang and J. Zhu (2015)." Towards High Energy Density Lithium Battery Anodes: Silicon and Lithium", Journal of Nano Lett. 15, 5750-5754. https://doi.org/10.1021/acs.nanolett.5b01698.
Baolin Xing, Huihui Zeng, Guangxu Huang, Chuanxiang Zhang, Ruifu Yuan, Yijun Cao, Zhengfei Chen, Jianglong Yu (2019). "Porous Graphene Prepared from Anthracite as Hig- Performance Anode Materials for Lithium-Ion Battery Applications", Journal of Alloys and Compounds, 779, 202-21. https://doi.org/10.1016/j.jallcom.2018.11.288
Bin Huang, Xinhai Li, Yi Pei, Shuang Li, Xi Cao, Robert C. Massé, and Guozhong Cao (2016). "Novel Carbon-Encapsulated Porous SnO 2 Anode for Lithium-Ion Batteries with Much Improved Cyclic Stability", Journal of Materials View, Small, 12 (14), 1945-1955. https://doi.org/10.1002/smll.201503419 .
Bin Wang, Chuangang Hu and Liming Dai (2016). "Functionalized Carbon Nanotubes And Graphene-Based Materials For Energy Storage", Journal of Chem. Commun., The Royal Society of Chemistry, 52, 14350-14360. https://doi.org/10.1039/C6CC05581H.
C. J. Wen and R. A. Huggins (1981). "Electrochemical Investigation of the Lithium‐Gallium System", Journal of Electro chem. Soc., 128, 1636-1641. https://doi.org/10.1149/1.2127701.
Cheng Lin, Aihua Tang, Ningning Wu, and Jilei Xing (2016). "Electrochemical and Mechanical Failure of Graphite-Based Anode Materials in Li-Ion Batteries for Electric Vehicles", Journal of Chemistry Hindawi Publishing Corporation, Volume, Article ID 2940437, 7 pages. http://dx.doi.org/10.1155/2016/2940437.
D-Y. Shin, K-W. Sung, H-J. Ahn (2020). "Fluorine-Doped Carbon Quantum Dot Interfacial Layer on Stockade-Like Etched Copper Foil for Boosting Li-Ion Storage", Journal of Chemical Engineering. https://doi.org/10.1016/j.cej.2020.127563.
D. Tang, J. Liu, X. Wu, R. Liu, X. Han, Y. Han, H. Huang, Y. Liu, Z. Kang (2014). "Carbon Quantum Dot/Nife Layered Double-Hydroxide Composite as a Highly Efficient Electro Catalyst for Water Oxidation". Journal of ACS Appl. Mater. Interfaces, 6, 7918. https://doi.org/10.1021/am501256x.
D. Wang, C. Zhou, B. Cao, Y. Xu, D. Zhang, A. Li, J. Zhou, Z. Ma, X. Chen, H. Song (2019). "One-Step Synthesis of Spherical Si/C Composites with Onion-Like Buffer Structure As High-Performance Anodes for Lithium-Ion Batteries", Journal of Energy Storage Materials. https://doi.org/10.1016/j.ensm.2019.07.045.
Deng, D., M. G. Kim, J. Y. Lee, and J. Cho. (2009). "Green Energy Storage Materials: Nanostructured Tio2 and Sn-Based Anodes for Lithium-Ion Batteries", Journal of Energ. Environ. Sci. 2, 818-837. https://doi.org/10.1039/b823474d .
En Qi, Joseph G. Shapter, Qian Wu, Ting Yin, Guo Gao and Daxiang Cui (2017). "Nanostructured anode Materials for Lithium-Ion Batteries: Principle, Recent Progress and Future Perspectives", Journal of Materials Chemistry-A. https://doi.org/10.1039/C7TA05283A .
F. Luo, B. Liu, J. Zheng, G. Chu, K. Zhong, H. Li, X. Huang and L. Chen (2015). " Ni-Sn Intermetallics as Efficient Buffering Matrix of Si Anodes In Li-Ion Batteries", Journal of Electro chem. Soc., 162, A2509-A2528. https://doi.org/10.1149/2.0131514jes .
Fu J, Liu H, Liao L, Fan P, Wang Z,Wu Y, Zhang Z, Hai Y, Lv G, Mei L,Hao H, Xing J and Dong J (2018). "Ultrathin Si/CNTs Paper-Like Composite for Flexible Li-Ion Battery Anode with High Volumetric Capacity", Jorunal of Frontier in Chemistry, 6, 624. https://doi.org/10.3389/fchem.2018.00624.
H. Li, X. He, Y. Liu, H. Huang, S. Lian, S.T. Lee, Z. Kang (2011). "One-Step Ultrasonic Synthesis of Water-Soluble Carbon Nanoparticles with Excellent Photoluminescent Properties". Journal of Carbon, 49, 605. https://doi.org/10.1016/j.carbon.2010.10.004.
H. Lu, R. Chen, Y. Hu, X. Wang, Y. Wang, L. Ma, G. Zhu, T. Chen, Z. Tie, Z. Jin and J. Liu (2017). "Bottom-Up Synthesis of Nitrogen-Doped Porous Carbon Scaffolds For Lithium And Sodium Storage" Journal of Nanoscale. https://doi.org/10.1039/C6NR08296C .
H.M.El Sharkawy, A.S. Dhemees, A.R. Tammen, S.M.El. Sabagn, R.M. Aboushabha, N.L.Allam (2020). " N-Doped Carbon Quantum Dots Boost the Electrochemical Supercapacitive Performance and Cyclic Stability Of Mos2", Journal of Energy Storage, 27, 101078. https://doi.org/10.1016/j.est.2019.101078.
Hao Zhang, Yang Yang, Dongsheng Ren, Li Wang, Xiangming He (2021). "Graphite as Anode Materials: Fundamental Mechanism, Recent Progress and Advances", Energy Storage Materials, 36, 147-170. https://doi.org/10.1016/j.ensm.2020.12.027.
I.-s. Kim, G. E. Blomgren and P. N. Kumta (2004). "Si-SiC Nanocomposite Anodes Synthesized Using High-Energy Mechanical Milling", Journal of Power Sources, 130, 275-280. https://doi.org/10.1016/j.jpowsour.2003.12.014 .
Issatayev, N.; Kalimuldina,G.; Nurpeissova, A.; Bakenov, Z (2022). "Biomass-Derived Porous Carbon from Agar as an Anode Material for Lithium-Ion Batteries". Nanomaterials, 12, 22. https://doi.org/10.3390/nano12010022 .
J. S. Bridel, T. Aza¨ıs, M. Morcrette, J. M. Tarascon and D. Larcher (2010). "Vinyltriethoxysilane Crosslinked Poly(Acrylic Acid Sodium) as a Polymeric Binder for High Performance Silicon Anodes in Lithium Ion Batteries", Journal of Chem. Mater. 22, 1229-1241. https://doi.org/10.1039/c8ra04967j .
J. Wang, I. D. Raistrick and R. A. Huggins(1986). "Behavior of Some Binary Lithium Alloys as Negative Electrodes in Organic Solvent-Based Electrolytes", Journal of Electro chem. Soc. 133, 457-460. https://doi.org/10.1149/1.2108601.
J. Wang, R. Sheng Li, H. Zhi Zhang, N. Wang, Z. Zhang, C.Z. Huang (2017). "Highly Fluorescent Carbon Dots as Selective and Visual Probes for Sensing Copper Ions in Living Cells Via an Electron Transfer Process", Journal of Biosens. Bioelectron, 97, 157. https://doi.org/10.1016/j.bios.2017.05.035 .
J. Zhu, D. Lei, G. Zhang, Q. Li, B. Lu, T. Wang (2013). "Carbon and Graphene Double Protection Strategy to Improve The Snox Electrode Performance Anodes for Lithium-Ion Batteries", Journal of Nanoscale, 5, 5499-5505. https://doi.org/10.1039/c3nr00467h.
J.Tarascon, P. Poizot, S. Laruelle, S. Grugeon and L. Dupont (2000). "Nano-sized Transition Metal Oxides as Negative Electrode Materials for Lithium Ion Batteries", Journal of Nature, 407, 496-499. https://doi.org/10.1038/35035045 .
Jiantie Xu, Yi Lin, John W. Connell, and Liming Dai (2015). "Nitrogen-Doped Holey Graphene as an Anode for Lithium-Ion Batteries with High Volumetric Energy Density and Long Cycle Life", Journal of small, 11(46), 6179-6185. https://doi.org/10.1002/smll.201501848.
Jiaqi Guo, Fangliang Gao, Dongyang Li, Xingjun Luo, Yiming Sun, Xingfu Wang, Zhilin Ran, Qibao Wu, and Shuti Li (2020). "A Novel Strategy of Constructing Hollow Ga2O3@N-Cqds As Self-Healing Anode Material for Lithium-Ion Batteries", Journal of ACS Sustainable Chemistry & Engineering, 8, 36, 13692–13700. https://doi.org/10.1021/acssuschemeng.0c03756 .
Jinhui Xu, Qingyang Yin, Xinru Li, Xinyi Tan, Qian Liu, Xing Lu, Bocheng Cao, Xintong Yuan, Yuzhang Li, Li Shen, and Yunfeng Lu (2022). "Spheres of Graphene and Carbon Nanotubes Embedding Silicon as Mechanically Resilient Anodes for Lithium-Ion Batteries", Nano Letters, 22 (7), 3054-3061. https://doi.org/10.1021/acs.nanolett.2c00341.
Jongmin Kim, Wooree Jang, Ji Hoon Kim, Cheol-Min Yang (2021). "Synthesis of Graphene Quantum Dots-Coated Hierarchical Cuo Microspheres Composite for Use as Binder-Free Anode for Lithium-Ion Batteries", J. of Elsevier Composites Part B 222, 109083. https://doi.org/10.1016/j.compositesb.2021.109083.
K. Kierzek, J. Machnikowski and F. B'eguin (2015). "High-capacity Group-IV Elements (Si, Ge, Sn) Based Anodes for Lithium-Ion Batteries" Journal of Appl. Electrochem, 45, 1-10. https://doi.org/10.1016/j.jmat.2015.06.002 .
KH Kim, HJ Ahn (2022). "Surface Functional Group‐Tailored B and N Co Doped Carbon Quantum Dot Anode for Lithium‐Ion Batteries", International Journal of Energy Research - Wiley Online Library. https://doi.org/10.1002/er.7738 .
L. Li, C. Lu, S. Li, S. Liu, L. Wang, W. Cai, W. Xu, X. Yang, Y. Liu, R. Zhang (2017). "A High Yield and Versatile Method for the Synthesis of Carbon Dots for Bioimaging Applications", Journal of Mater. 5, 1935. https://doi.org/10.1039/C6TB03003C .
L. Xu, Y. Tian, T. Liu, H. Li, J. Qiu, S. Li, H. Li, S. Yuan, S. Zhang (2018). "α-Fe2O3 Nanoplates With Superior Electrochemical Performance for Lithium-Ion Batteries", Journal of Green Energy & Environment. https://doi.org/10.1016/j.gee.2018.01.005.
Li Z, Cui Y, Chen J, Deng L, Wu J (2016). "Fabrication of (Co,Mn)3O4/rGO Composite for Lithium Ion Battery Anode by a One-Step Hydrothermal Process with H2O2 as Additive", Journal of PLoS ONE, 11(10), e0164657. https://doi.org/10.1371/journal.pone.0164657.
M. Javed, A.N.S. Saqib, Ata-ur-Rehman, B. Ali, M. Faizan, D.A. Anang, Z. Iqbal, S.M. Abbas (2018). "Carbon Quantum Dots From Glucose Oxidation as a Highly Competent Anode Material for Lithium and Sodium-Ion Batteries", Journal of Electro chimica Acta. https://doi.org/10.1016/j.electacta.2018.11.167.
M. Jing, J. Wang, H. Hou, Y. Yang, Y. Zhang, C. Pan, J. Chen, Y. Zhu and X. Ji (2015). "Carbon Quantum Dots Coated Mn3O4 with Enhanced Performances for Lithium-ion Batteries", Journal of Materials Chemistry- A, 3, 16824-16830. https://doi.org/10.1039/C5TA03610K.
N. Gao, L.Huang, T.Li, J.Song, H.Hu, Y.Liu, S.Ramakrishna (2019). "Applications of Carbon Dots in Dye-Sensitized Solar cell: a review", Journal of Applied Polymaer Science, 48443. https://doi.org/10.1002/app.48443.
Naoki Nitta, Feixiang Wu, Jung Tae Lee and Gleb Yushin (2015). "Li-ion battery Materials: Present and Future", Journal of Materials Today, 18(5) 38, 275 - 283 275. https://doi.org/10.1016/j.mattod.2014.10.040.
Peng Guo, Huaihe Song, Xiaohong Chen (2009). "Electrochemical performance of Graphene Nanosheets as Anode Material for Lithium-Ion Batteries", Journal of Electrochemistry Communications, 11, 1320-1324. https://doi.org/10.1016/j.elecom.2009.04.036.
Q. Huang, H. Zhang, S. Hu, F. Li, W. Weng, J. Chen, Q. Wang, Y. He, W. Zhang, X. Bao (2014). "A Sensitive And Reliable Dopamine Biosensor was Developed Based on the Au@Carbon Dots-Chitosan Composite Film". Journal of Biosens. Bioelectron, 52, 277. https://doi.org/10.1016/j.bios.2013.09.003.
Qi, Wen, Joseph G. Shapter, Qian Wu, Ting Yin, GuoGao, and Daxiang Cui. (2017). "Nanostructured Anode Materials for Lithium-Ion Batteries: Principle, Recent Progress and Future Perspectives", Journal of Materials Chemistry A, 5, no. 37, 19521-19540. https://doi.org/10.1039/C7TA05283A.
Qingke Tan, Zhen Kong, Xiaojing Chen, Lei Zhang, Xiaoqi Hu, Mengxin Mu, Haochen Sun, Xinchun Shao, Xianggang Guan, Min Gao, Binghui Xu (2019). "Synthesis of SnO2/Graphene Composite Anode Materials for Lithium-Ion Batteries", Journal of Applied Surface Science, 485, 314-322. https://doi.org/10.1016/j.apsusc.2019.04.225.
R. Liu, W. Su, P. He, C. Shen, C. Zhang, F. Su, C.-A. Wang (2016). "Synthesis of SnO2/Sn Hybrid Hollow Spheres as High-Performance Anode Materials for Lithium-Ion Battery", Journal of Alloys and Compounds. https://doi.org/10.1016/j.jallcom.2016.07.194.
S. Wang, H. Wang, R. Zhang, L. Zhao, X. Wu, H. Xie, J. Zhang, H. Sun (2018). "Egg Yolk-Derived Carbon: Achieving Excellent Fluorescent Carbon Dots and High-Performance Lithium-Ion Batteries", Journal of Alloys and Compounds. https://doi.org/10.1016/j.jallcom.2018.02.293.
S. Yang, P. He, T.Yuan, Y. Li, X. Li, Y. Zhang, L. Fan, Y. Shi and T. Meng (2020). Recent Advances in White Light-Emitting Diodes of Carbon Quantum Dots", journal of Nanoscale. https://doi.org/10.1039/C9NR10958G.
S. Zhuo, M. Shao, S.T. Lee (2012). "Up Conversion and Down Conversion Fluorescent Graphene Quantum Dots: Ultrasonic Preparation and Photocatalysis". Journal of ACS Nano, 6, 1059. https://doi.org/10.1021/nn2040395.
Tian L L, Zhuang Q C, Li J, et al (2011). "Mechanism of Intercalation and Deintercalation of Lithium Ions in Graphene Nanosheets", Journal of Chinese Science Bulletin, 56(30), 3204-3212. https://doi.org/10.1007/s11434-011-4609-6 .
Tian-Bing Song, Zun-Hui Huang, Xiao-Qing Niu, Jun Liu, Ji-Shi Wei, Xiao-Bo Chen, and Huan-Ming Xiong (2020). "Applications of Carbon Dots in Next-generation Lithium-Ion Batteries", Chem Nano Mat 6, 1-17. https://doi.org/10.1002/cnma.202000355.
W. Ren, Y. Wang, Z. Zhang, Q. Tan, Z. Zhong and F. Su (2016). "Nanostructured Anode Materials for Lithium-Ion Batteries: Principle, Recent Progress and Future Perspectives", Journal of Appl. Surf. Sci. 360, 192-197. https://doi.org/10.1039/C7TA05283A.
W. Ren, Z. Zhang, Y. Wang, G. Kan, Q. Tan, Z. Zhong and F. Su (2015). "Preparation of Porous Carbon Microspheres Anode Materials from Fine Needle Coke Powders for Lithium-Ion Batteries", Journal of RSC Adv. 5, 11115-11123. https://doi.org/10.1039/C4RA15321A.
W. Ren, Z. Zhang, Y. Wang, Q. Tan, Z. Zhong and F. Su (2015). "Preparation of Porous Silicon/Carbon Microspheres as High-Performance Anode Materials for Lithium-Ion Batteries" Journal of Mater. Chem. A. 3, 5859-5865. https://doi.org/10.1039/C4TA07093C.
Wei Zhang, Sheng Fang, Ning Wang, Jianhua Zhang, Bimeng Shi, Zhang long Yu and Juanyu Yang (2020). "A Compact Silicon-Carbon Composite with an Embedded Structure for High Cycling Coulombic Efficiency Anode Materials in Lithium-Ion Batteries", Journal of Inorganic Chemistry Frontiers. https://doi.org/10.1039/D0QI00302F.
Weimin Zhao, Jingjing Wen, Yanming Zhao, Zhifeng Wang, Yaru Shi, and Yan Zhao (2020)."Hierarchically Porous Carbon Derived from Biomass Reed Flowers as Highly Stable Li-Ion Battery Anode Nanomaterials". 10, 346. https://doi.org/10.3390/nano10020346.
X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens (2004). "Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments", Journal of American Chemical Society, vol 126, 12736. https://doi.org/10.1021/ja040082h.
X. Yin, C. Zhi, W. Sun, L. Lv and Y. Wang (2019). "Multilayer NiO@Co3O4@Graphene Quantum Dots Hollow Spheres for High-Performance Lithium-Ion Batteries and Supercapacitors", Journal of Materials Chemistry A. https://doi.org/10.1039/C8TA11982A.
X.T. Zheng, A. Anantha narayanan, K.Q. Luo, P. Chen (2015). "Glowing Graphene Quantum Dots And Carbon Dots: Properties, Syntheses, and Biological Applications". Journal of Small, 11, 1620. https://doi.org/10.1002/smll.201402648.
Y. Zhang, L. Jiang, C. Wang (2015). "Facile Synthesis of SnO2 Nanocrystals Anchored onto Graphene Nanosheets as anode materials for Lithium-ion batteries", Journal of Phys. Chem, 17, 20061-20065. https://doi.org/10.1039/C5CP03305E
Y. Zhang, L. Jiang, C. Wang (2015). "Facile Synthesis of Sno2 Nanocrystals Anchored Onto Graphene Nanosheets as Anode Materials for Lithium-Ion Batteries", Journal of Phys. Chem., 17, 20061-20065. https://doi.org/10.1039/C5CP03305E.
Yangzhi Bai, Xinlong Cao, Zhanyuan Tian, Shifeng Yang, Guolin Cao (2021). "A High-Performancesilicon/Carbon Composite as Anode Material for Lithium-Ion Batteries", Journal of Nano Express, IOP Publishing. https://doi.org/10.1088/2632-959X/abdf2e.
Zhanwei Xu, Yixing Zhao, Juju He, Tian Wang, Jun Yang, Xuetao Shen, Liyun Cao, and Jianfeng Huang (2019). " MoO3/Carbon Dots Composites for Li-Ion Battery Anodes", Journal of Chem Nano Mat. https://doi.org/10.1002/cnma.201900140.
Zhuo Deng, Tingting Liu, Tao Chen, Jiaxiang Jiang, Wanli Yang, Jun Guo, Jianqing Zhao, Haibo Wang, and Lijun Gao (2017). "Enhanced Electrochemical Performances of Bi2O3/rGO Nanocomposite via Chemical Bonding as Anode Materials for Lithium-Ion Batteries", Journal of ACS, Applied Materials and Interfaces. https://doi.org/10.1021/acsami.7b00996.
Zihao He, Jing Huang, Kun Liu, Xuekun Tang, Ying Dai & Guozhao Fang (2022). "Construction of Graphitic Carbon Quantum Dots-Modified Yolk-Shell Co3O4 Microsphere for High-Performance Lithium Storage", Journal of Materials Science, 57, 3586-3600. https://doi.org/10.1007/s10853-021-06814-0.
|
|
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© IJETMR 2014-2022. All Rights Reserved.


