ShodhKosh: Journal of Visual and Performing ArtsISSN (Online): 2582-7472
|
|
The Blue Green Patina on Sculptures Cast in Copper and Its Alloys - Their Chemistry and Aesthetics
Aashu Chawla 1![]() , Dr. Giriraj Sharma 2
, Dr. Giriraj Sharma 2![]()
![]()
1 Research
Scholar, Fine Art Department, IIS (Deemed to be University), Jaipur, Rajasthan,
India
2 Associate Professor, Fine Art Department, IIS (Deemed to be University), Jaipur, Rajasthan India
|
|
ABSTRACT |
||
|
Copper and its
alloys have a tendency to naturally corrode and form
a blue green finish over them. This blue green finish has been observed in
everyday copper and bronze utensils and even in famous sculptures like statue
of liberty. Artists have long taken advantage of this patina to achieve
aesthetic of desolateness, age, and damage. This patina tends to change the luster
of the metal from reflective to matt and color from pink (gold in case of
brass and bronze) to bright blue or green. Artists are known to use this
change to add to visual language of their sculptures. The current paper takes
in account the whole spectrum of blue – green patinas, from bright blue
azurites to dull green verdigris and also dark green
copper chloride salts. The paper discusses the chemistry behind the formation
of this patina, tests age old recipes and discusses aesthetics with respect
to famous Indian sculptors. |
|||
|
Received 22 March 2022 Accepted 28 April 2022 Published 16 May 2022 Corresponding Author Aashu Chawla, DOI 10.29121/shodhkosh.v3.i1.2022.108 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2022 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Patina, Verdigris, Sculpture, Copper Rust,
Aesthetics |
|||
1. INTRODUCTION
Corrosive
properties of copper had been known to man ever since the discovery of copper.
Copper and its alloys, if not protected, rust under environmental conditions.
The conditions determine the compound formation on metal surface and these
compounds occur in various brilliant colors and luster. Ancient sculptors took
notice of this phenomenon and tried to experiment with it. Where ancient
Egyptians focused on developing wax and polishes to avoid metal tarnishing,
Ancient Greeks and Asians through trial and error introduced recipes to make
even patina finishes all over their works. A lot was talked about of the
aesthetic of rust and corrosion in Japan and China as well. There have been
many instances through ancient history where byproducts of corrosion or direct
corroded copper have been employed as pigments or even medicine. This
fascination with changes that occur through age and weathering was extensively
studied and listed under an umbrella term of Patina.
Patina
has been studied in various fields like chemistry, conservation sciences,
environmental practices, and even aesthetics and fine arts. Even after
extensive research, no comprehensive study on artificial patina on sculptures,
discussing its chemistry, recipes and how it affects the aesthetics of the
sculpture seems to have been worked upon. Separate efforts have been made to
explain chemistry of corrosion in both scientific and conservation fields.
Researchers like C. Leygraf, J. P. Franey, have extensively studied the compounds formed after
corrosion that produce various colors on copper Leygraf et al.
(2019), Franey and
Davis (1987). Papers have also been written on
age old recipes and processes of blue green patina by the likes of David Scott
and Mary Virginia Orna (Mary Virginia Orna, 1980) Scott et
al. (2001). Aesthetics of Patina have also
been talked about in philosophical terms in books and papers by Manuel
Ortega-Calvo, Koren, and Silva (Manuel Ortega-Calvo,
2014) Koren (2008) Silva et
al. (2017). The present paper focuses on
techniques, recipes, and aesthetics of natural and artificial blue green patina
on contemporary sculptures made in copper and its alloys.
There
were several issues in installing sculptures with virgin metal surfaces and
letting the environment take its course with it. Firstly, there was no way of
controlling the thickness, color, and texture of the patina. This created a
risk of patina turning vile. Secondly, artists had limited options of patina
that the specific environmental conditions could naturally produce. Natural
patinas can be spatially heterogeneous, meaning they are neither uniform nor
smooth. In addition to that, each sculptural surface with its crevices, nooks,
cuts, bends, and folds will interact differently with the environment. Varying
degrees of sunlight exposure is also a contributing factor of non-uniform
patina Graedel (1987). Therefore, sculptors prefer to
produce these surfaces artificially before letting nature take its course.
Artificial patina produces an even layer; the artist can customize shadows and
highlights according to his preference. In some cases, artists have been
deliberately using artificial patina to form an uneven layer that adds to the
language of the sculpture.
Copper and its alloys, brass and bronze have
been the prime choice of medium for sculpting since ancient times. The choice
is attributed to its malleability and ductility, low melting point and
strength. Another important choosing factor especially for modern sculptors and
architects is its ability to form patina Graedel (1987). Post modern and contemporary eras
saw artificial recipes being developed to produce these corroded surfaces in
colors other than the usual, brown, green, and black. However, it was observed
that most environmental conditions lead copper to inevitably turn into the
iconic blue green patina. The blue green patina once established is relatively
stable. When studied under spectroscopy the patina was known to be a mixture of
oxides, sulfates and carbonates and had many variants in compositions discussed
under section 2 of the paper.
Different
colorful effects had been applied to bronze sculptures since early classical
times, but it was not until the eighteenth century that green corrosion
products began to be of value. According to the Oxford Dictionary of the English
Language, the earliest reference to the use of green patina dates from 1797 Koren (2008). Blue green patina also became
popular when ancient artists and sculptors found out that exposure to acetic
acid makes copper turn green relatively quickly. A blanket term for this acetic
acid produced blue-green patinas is known as verdigris – Vert de Grece roughly translated to Green of Greece, or the French
spelling Vert de gris translated to green of grey. Verdigris is known as both
the patina as well as the pigment that is scraped off the copper to be later
used in paintings Scott
(2002). The accessibility of materials and
ease of the process with which this patina can be achieved made it popular
amongst the masses.
Another
aspect of popularity is familiarity with the color, since this color is
naturally achieved on copper objects around in everyday life like utensils,
vessels, bathroom amenities, pipes and tools if left without care. This made
verdigris come to be associated with old, desolated things and antiques. Soon
artists discovered ways to recreate this finish artificially, taking advantage
of the familiarity and the color’s association with the age. Artists found
philosophical references with the ever-changing patina which is in artistic
terms referred to as a live patina. It becomes imperative to discuss how this
alteration affects the language of the sculpture.
1.1. OBJECTIVE
The
current paper aims to understand the chemistry of the compounds and
environmental conditions that cause copper and its alloys to acquire a blue
green finish. It also discusses the methods of recreating the same finishes on
sculptures artificially. Lastly it aims to observe and analyze various modern
sculptures in which artists have employed this patina finish to their
advantage.
2. CHEMISTRY FOR PATINATION
In terms of metal cast sculptures (especially those cast in copper and its alloys), the environment where they are installed affects the composition of the patina on them. In most cases, virgin copper first interacts with oxygen, creating copper oxides which are reddish brown to dark brown in colour. In presence of sulfur or sulfur dioxide, and humidity in the environment, Oxides turn to sulfate minerals that give the copper its iconic blue green colour. Depending on the actual environment and exposure time, the sulfate minerals that form on copper surface can be posjnakite (Cu4SO4(OH)6.H2O), brochantite (Cu4SO4(OH)6), antlerite (Cu3(SO4) (OH)4), atacamite (Cu2Cl (OH)3) or combinations thereof (C. Leygraf, 2019). Antlerite, brochantite, and chalcanthite are the dominant corrosion products formed during the extended atmospheric exposure of copper and copper alloys Noli et al. (2003).
Copper sulphate is popularly known as blue vitriol. Ninth
century Indian physician and writer Vagbhata is known
to have used the term Sasyaka in Sanskrit for the
same. It is one among the Maharasa (group of minerals)
in Ayurveda and finds a huge significance in Ancient Indian medicine. Naturally
occurring copper sulphate is called Sasyaka while the
artificially made is termed Tuttha. Chemically the
formulas differ, Sasyaka is Cu5FeS4 and Tuttha is CuSO4.5H2O. Tuttha
is known as copper (II) sulphate, (chalcanthrite),
blue vitrol and blue stone and it is obtained through
processing the sulphuric acid over the copper. Sasyaka
is known as naturally occurring copper iron sulphide, which reflects the colour
similar to the neck of a peacock and is heavy in
weight Shankar
(2019).
Brochantite
is the most stable and most often occurring patina of copper alloys in the
environment and has a greener finish than the blue of chalcanthrite.
Antlerite is another commonly occurring copper sulphate mineral and has a
similar vitreous green finish. These kinds of patinas were found to occur
naturally on both Lorenzo Ghiberti’s Gates of Paradise and Rodin’s Thinker Scott
(2002).
Copper is
known to form salts in reaction with various organic acids like acetic acid,
formic acid, tartaric acid, and citric acid. These acids can be sourced from
plants, fruits, resins, urine etc. As discussed above in Section 1, these
patinas form verdigris and a bundle of ancient recipes from all around the
world can be found to produce these. Since medieval times, a patina pit was a
common occurrence in metal foundries, where cast sculptures were buried with
products that are known to produce acetic acid. Lemons, grapes, pineapple,
vinegar and wine, salt and honey and ammonia from urine are known to be used in
producing verdigris. This experiment has been conducted under section 3.2.
Verdigris ranges from pale blue through turquoise till green and is extremely
difficult to consistently produce the exact same results every time. The
recipes rely on naturally occurring products which makes it difficult to
control external factors and compounds that seep in along with the desired
acetic acid. As a pigment, verdigris is typically greenish blue
when it is first applied and develops its full
green hue after a month following application Roy (1986).
Excess carbon in the air or dissolved carbon in waters around copper result in basic copper carbonate. More accurately known as copper carbonate hydroxide Cu2CO3(OH)2. It occurs in nature most readily as Malachite and is often found in areas with copper deposits near limestones (CaCO3) or other carbonic sources. Another similar mineral is Azurite, it can form readily in natural environments if very dilute CuSO4 solutions react with Ca (HCO3)2 solutions, which usually result from the dissolution of limestone by CO2-rich waters. Where Azurite is deep blue in colour, Malachite is bright green to blackish green. Both exist in forms of crystals on top of the surface of copper and are very rarely vile. Widely used as the green pigment, colouring agent in glazes and glass making, it occurs often as a corrosion product on buried bronzes. Small crystals of azurite can be produced by rapidly stirring a few drops of copper sulfate solution into a saturated solution of sodium carbonate and allowing the solution to stand overnight Scott et al. (2001)
Darker green patina is formed due to copper
chloride compounds. Atacamite and its polymorphs Cu2(OH)3Cl,
nantokite CuCl, antlerite Cu3(OH)4SO4
form green, and it can be lightened by lead carbonate PbCO3 or tin
oxide SnO2 (G.Di Carlo, 2017). Chloride
patinas are usually found in artifacts that are discovered from underwater
areas or areas with very moist air. Artists usually stay away from chloride as cupric
chloride has a tendency to turn vile. Commonly
referred to the ‘Bronze Disease’, chloride corrosion
on copper-based artifacts is highly contagious and eats away the metal. To preserve sculptures with bronze disease, they
are to be kept away from oxygen, water, and chlorides. Differentiation of verdigris from bronze disease
can be made using two
pointers. Verdigris is pale green where chloride compounds are bright to dark
green. Verdigris, as mentioned above, is highly stable, where bronze disease
can be scraped off using a
wooden stick or nail thus making the sculpture’s layer weaker with its action Turner-Walker (2008).
3. METHODOLOGY
3 by 4
inches and 4 mm thick copper and brass slides were taken for experimentation.
The slides were washed and sanded off with first a soft steel wool and then 620
grit sandpaper. This was done to remove a layer of oxidation on top and bring
the virgin metal forward.
Hot
Process: A heat gun
was aimed at the slide, to achieve a temperature of 125-130 degrees. Chemicals
were then sprayed on the slide. The moisture in the chemicals evaporates
immediately on hitting the slide and chemicals start to act on the virgin
metal. The spray has to be even and thin in order to
achieve a long-lasting patina. In case of increase in temperature of the heat
gun held too close to the slide, the patina will burn to form splotchy black
spots.
Cold
Process: The slide
was either submerged in or painted upon with chemicals at room temperature for
the given duration in the recipe. The chemicals work relatively slower that the
hot process and the patina is often uneven. A relatively closed environment was
achieved where impurities like dust, pollution etc. were kept to minimum.
3.1. EXPERIMENT WITH APPLE CIDER VINEGAR AND TABLE SALT
Two
plates of copper and brass were left submerged in a solution of 90 mL apple
cider vinegar and 30 grams of iodized salt. The observations are as follows.
·
Day 1– Slight
brightness on the metal, copper is in its original pink color and brass is its
original yellow. The slight oxidization and impurities seem to get removed Figure 1.
Figure 1

|
Figure 1 Copper (Left) And Brass (Right) Submerged In A Solution Of Apple Cider Vinegar And Salt |
·
Day 7– No
change
·
Day 14–
Start to see slight browning in both copper and brass.
·
Day 21–
Green crystals start to form at the edge of the tray and on the edges of the
plates.
·
Day 28–
Crystallization process starts rapidly. The plates turn bright green, with
crystals forming on the edges as the vinegar volume starts to decrease Figure 2.
Figure 2

|
Figure 2 Copper (Left) And Brass (Right) Copper Acetate (Verdigris) Crystals Form as Vinegar Solution Gets Absorbed Off Completely |
·
Day 32– The
plates have been covered with verdigris crystals; vinegar solution has
completely evaporated leaving behind the whole tray with dark green to green
crystals Figure 3 Figure 4.
Figure 3

|
Figure 3 Brass Plate with Dark Green Verdigris Crystal Formation |
Figure 4

|
Figure 4 Details of Scraped Off Crystals of Verdigris |
3.2. COPPER NITRATE EXPERIMENTS
Hot
process was opted to execute this patina. A spray of a water diluted solution
of copper nitrate was sprayed evenly on the brass slide. The composition of the
chemical should depend on the patina artist and how opaque the color needs to
be. Sky blue color appears after 2-3 sprays. This patina is highly sensitive to
fire and often gets burnt. The recipe works on all copper alloys.
Figure 5
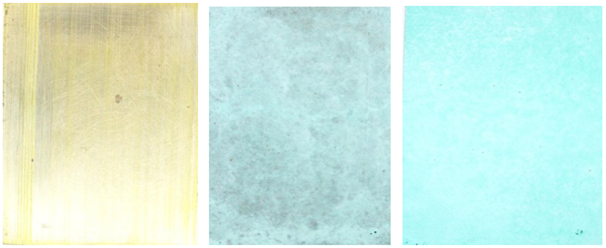
|
Figure 5 a) Brass Plate Washed and Lightly Abrade to Remove Any Oxidation. b) Brass Plate With 3-4 Light Sprays Of 3:7 Ratio of Copper Nitrate and
Water. (Hot Process) c) Brass Plate With 6-8 Light Sprays Of 4:6 Ratio of Copper Nitrate and
Water. (Hot Process) |
3.3. COMBINATION OF COPPER SULFATE AND FERRIC NITRATE
Dull sea
green to yellow sap green can be produced by mixing the two solutions: Copper
Nitrate and Ferric Nitrate. Hot process of patination is used to spray thin
coats of the solution. Experimentation with ratios of chemicals will result in
various tones of green.
Figure 6
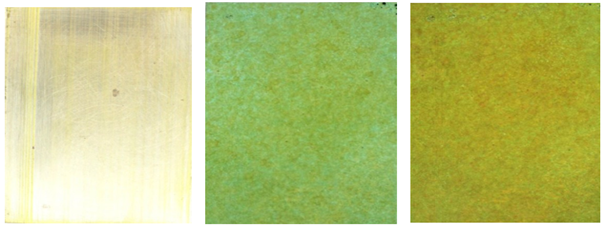
|
Figure 6 a) Brass Plate Washed and Lightly Abrade to Remove Any Oxidation. b) Brass Plate First Sprayed 3-4 Times with Copper Sulfate Solution in Water In 3:7 Ratio. Then Ferric Nitrate Solution with Water of Ratio 2:8 Is Sprayed on Thinly Twice. c) Brass Plate First Sprayed 3-4 Times with Copper Sulfate Solution in Water In 3:7 Ratio. Then Ferric Nitrate Solution with Water of Ratio 2:8 Is Sprayed on Thinly 4-5 Times. |
3.4. AMMONIA EXPERIMENTS
This
experiment was conducted using two different methods, cold fuming, and cold
submerging process. Ammonia in itself produces a light
green color, but during literature review it was found that salt is used to
encourage better stability of the ammonia layer.
Slides
were sprinkled with water and then drops of ammonia were poured on the slide.
The patina thus formed was thicker and opaque. Could be used to add extra
texture to the metal but might not be feasible for sculptures with no flat
surfaces.
Figure 7

|
Figure 7 a) Brass Plate. Washed And Slightly Abrade to Remove Oxidation. b) Pure Ammonia Is Pooled Over the Slide and Crystals of Salt Are Sprinkled Over. c) Formation Of Whitish Blue Patina with Edges Turning Green. d) Copper Plate. Washed And Slightly Abrade to Remove Oxidation. e) Pure Ammonia Is Pooled Over and Salt Crystals Are Sprinkled on Top. f) Green Patina Is Seen to Develop with Some Areas Are Observed to Be Greenish Brown. |
4. AESTHETICS
Aesthetics
in sculpture can be divided into two broad categories, beauty of sight and
beauty of touch. We do not appreciate sculpture by touching them but rather by
seeing them. Modern sculptures are usually kept under protection of museums or
even if displayed in public places are not accessible to touch. Therefore,
aestheticians coined the term imaginative touch, sense of sight as guided by
touch or as a substitute for touch. This is why
sculptors pay very careful attention to the textures of their forms. A rough
texture of a cement sculpture evokes a different response from the observer
than a highly polished aluminum even if the form is exactly
the same. Patina is used as a brilliant tool to add texture over a metal
sculpture as well as play with the luster of the metal. Patina can be used to
enhance the form of the sculpture, i.e., darken the depth in the sculpture or
add a lighter tone to the highlights. Sculptors use multiple colors of patina
to create tonal variation on a monotonous surface. Artificial patina can also
help to create harmony in an artwork. Artist Laxma
Gaud almost extensively uses a blue green patina on a base of brown for the
purpose of highlighting the desired area. The shades of green in his work
enhance the simulated and real textures of the form. It also harmonizes all the
different forms and textures into a one holistic visual.
Figure 8

|
Figure 8 Untitled
by Laxma Gaud, Bronze, 7” X 5” X 5” |
In the
Stanford encyclopedia of philosophy, under the section of Japanese aesthetics,
the word ‘Sabi’ refers to rustic patina Zalta et al.
(1995). Earlier the interpretation of the
term was associated with the feelings of dejection, loneliness, and neglect Helmenstine
(2020). There was a connotation of
desolateness attached to this concept. One of the prime reasons for the patina
being formed on a sculpture is neglect. If the sculpture is kept untouched and
unmaintained for an extended period of time, it
develops a patina that might turn vile. In terms of visual language, this kind
of patina will add a sense of gloom and detachment. As in the case of ‘Gandhi’s
three monkeys’ by Subodh Gupta where the artist uses blue green patina over a grey,
brown background. The blue green patina makes the soldiers look like they have
been stationed here for a long time at one place. Thus, a patina could be a
great way to make the sculpture look like it is a part of the selected
landscape it is to be installed in. The patina can make it look like the
sculpture has been in the position for an extended period of
time. This gives the sculptor the opportunity to establish a unique
connection with the environment.
Figure 9

|
Figure 9 ‘Gandhi’s Three Monkeys’ by Subodh Gupta, Bronze, Steel and Old Utencils, Doha, Quatar. Source : https://qm.org.qa/en/visit/public-art/subodh-gupta-gandhis-three-monkeys/ |
Later the
definition of Sabi was changed to something that has
aged well. A sense of beauty was associated with the effect of aging. Patina
meant ripe with experience and insight. Thus, patina on sculptures started to
be associated with not only peace and tranquility but also wisdom Koren (2008). The person watching a sculpture
with a blue green patina (even artificial) is tricked to believe that the
sculpture is older than him. This psychological trick can be played to
advantage when artists want to produce an image of a knowledgeable public
figure like Buddha or Gandhi. Ram Kinker Baij created a bust of his guru Rabindranath Tagore and
used blue green patina over matt black. The viewer is used to seeing blue green
patina as a highlight, but Ram Kinker Baij uses green in the depth making the wrinkles and
imperfections of the skin of his old model come to focus. This ages the
sculpture and its muse with insight.
Figure 10

|
Figure 10 ‘Bust of Rabindranath Tagore’ By Ram Kinker Baij. Bronze Source: NGMA, Delhi |
Raghav
Kaneria’s 1984 sculpture titled ‘Nandi’ has an even green patina with brown
undertones. The sculpture exhibits an earthy and dated quality that imbibes
within a personal and intuitive approach of the artist. Patina is used here to
negate the romanticization of technology that comes with a brightly polished
bronze surface. The aesthetic of blue green patina is in many ways antithesis
of what classical antiquity sculptures are known to
exhibit. The concept of perfection gets countered by the rustic simplicity. Soetsu believes that perfection in an artwork can be
limiting. In his book ‘Unknown Craftsman’, he writes that we in our human
imperfection are repelled by the perfect since everything is apparent from the
start and there is no suggestion of the infinite Yanagi et al.
(1989).
Figure 11

|
Figure 11 Nandi, Raghav Kaneria, Bronze, 14” X 10” X
8” Source: Scanned from Exhibition Catalogue, ‘Exhibition of Raghav Kaneria’, 2015 |
Natural
patina therefore was extensively observed and studied to be recreated in
studios artificially. Although nature will always be the benchmark, artists
started to play around with colors and textures to often achieve effects that
nature could never create. Artists realized that if the aesthetic of patina
needs to be preserved and celebrated there is a demand for guideposts and
scientific reasoning backing the craft for future generations to follow. Himmat
Shah’s 2006 Untitled work has a blue green patina over all in the sculpture
giving it an earthy, grounding quality. He buffs out the nose of the head,
adding to a visual contrast of luster. The sculpture creates a visual play of
bright and dull, reflective, and matt, polished, and rough.
Figure 12

|
Figure 12 Untitled
by Himmat Shah, Bronze, 12.5" X 7" X 6" Sanchit
Art Gallery, New Delhi |
The
aesthetic of the blue green patina is unique compared to the other patinas for
copper and its alloys. The blue green patina is the inevitable truth of any copper-based
object. It is the truth that time brings and that is why blue green patina can
be effective in showing that time has caught on with the sculpture and has
brought the metal to a state it is meant to be in a state of rest and balance.
Even though it is still changing, and time will still
continue to create its magic on the surface, this patina is relatively
stable and slow to change. Statue of liberty acts as a perfect example of how
blue green patina even though highly stable is relatively slower to establish.
When one observes the stages of evolution of its patina one realises
the concept of now, the statue was installed with shiny brown patina in 1886.
In 1906 it had completely transformed to the iconic blue green color we have
known to recognize it with. Presumably, it will not be the same in 50 more
years. Other than the fascination with the never-ending process, this also
makes the viewer appreciate the cosmic order and accept the inevitable Helmenstine
(2020). The wabi sabi
philosophy of ‘devolving towards and evolving from nothingness’ comes to play
here Koren (2008).
Figure 13
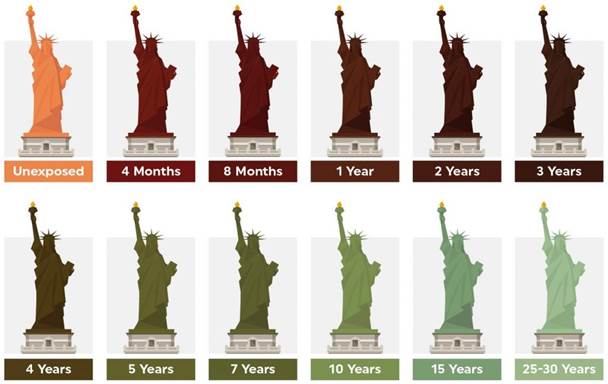
|
Figure 13 Creative Visualization of Patina Changing Over the Years on Statue of Liberty, New York Source: https://jtcroofing.co.uk/news/why-does-copper-turn-green/ |
5. Conclusion
It can be
safely concluded that the blue green patina has made its way from rust on
household items into a unique aesthetic in fine arts. Through experimentation
on the naturally occurring patina on copper, scientists found out that various
environmental factors act on copper to corrode it. Sulfur in the atmosphere
makes copper turn to copper sulfate salts, brochantite is most stable and has a
greener color where chalcanthite gives out a bright blue hue. Copper acetate
salts are made with exposure to acetic acid and are referred to as verdigris
and have a dull green-grey finish. Exposure to chlorine is considered dangerous
for copper and bronze sculptures, as copper chloride spreads quickly, eating
away the metal surface. This is darker green in color.
Artificially,
these patinas can be achieved using copper sulfate and ferric sulfate solutions
and a hot process. For cold processes, the sculpture surface needs to be
saturated with acetic acid from grapes, vinegar, wine etc. over time to produce
bright verdigris color. Another method is to pool ammonia and table salt over
the sculpture's surface to form an uneven and interesting blue green patina.
Observation of this patina in nature helped
artists recreate the feelings of imperfection, dejection, and add a rustic
charm to their sculpture artificially. When artists produce this patina
artificially on their sculptures it helps them achieve the effect of nature
taking its course over the sculpture, it tricks the viewer to believe the
artwork has been aged over centuries. This patina also helps artists enhance
textures of their works and add highlights. It was also seen that this patina
creates a great contrast against the original color and shine of the metal.
CONFLICT OF INTERESTS
None .
ACKNOWLEDGMENTS
None
.
REFERENCES
Franey, J. P., & Davis, M. E. (1987). Metallographic studies of the copper patina formed in the atmosphere. Corossion Science, 27(7), 659-668. https://doi.org/10.1016/0010-938X(87)90048-5
Graedel, T. E. (1987). Copper patina formed in the atmosphere I. Corrosion Science, 639-675. https://doi.org/10.1016/0010-938X(87)90047-3
Helmenstine, A. M. (2020). Why is the Statue of Liberty green ?
Koren, L. (2008). The Metaphor of Patina. In L. Koren (Ed.), Wabi-Sabi : For Artists, Designers, Poets & Philosophers. Imperfect Publishing.
Leygraf, C., Chang, T., Herting, G., & Odnevall Wallinder, I. (2019). The origin and evolution of copper patina colour. Corrosion Science, 157, 337-346. https://doi.org/10.1016/j.corsci.2019.05.025
Noli, F., Misaelides, P., & Hatzidimitriou, A. (2003). Investigation of artificially produced and natural copper patina layers. Journal of Materials Chemistry, 13(1), 114-120. https://doi.org/10.1039/b206773k
Roy, A. (1986). Artists' Pigments A Handbook of Their History and Characteristics. Washington Archetype Publications, London.
Scott, D. A. (2002). Copper and Bronze in Art - Corrosion, colorant, conservation (1st ed.). Getty Publications, Los Angeles.
Scott, D. A., Taniguchi, Y., & Koseto, E. (2001). The verisimilitude of verdigris: a review of the copper carboxylates. Studies in Conservation, 46, 73-91. https://doi.org/10.1179/sic.2001.46.Supplement-1.73
Shankar, P, P. (2019). Mineralogical Identification And Characterisation Of Sasyaka-an Ayurvedic Drug. International Journal of Ayurveda and Pharma Research, 75-78.
Silva, C., Vélez, G., & Colorado, H. A. (2017). Patina in the construction of the poetic bronze image : Science of materials, art and philosophy. Heritage Science, 5(1). https://doi.org/10.1186/s40494-017-0149-y
Turner-Walker, G. (2008). A Practical Guide to the Care and Conservation of Metals. Headquarters Administration of Cultural Heritage, Council for Cultural Affairs.
Yanagi, S. Yanagi, M, & Leach, B. (1989). The unknown craftsman: A Japanese insight into beauty. Kodansha International.
Zalta, E., Nodelman, U., & Allen, C. (1995). The Stanford Encyclopedia of Philosophy. The Metaphysics Research Lab, Philosophy Department, Stanford University.
|
|
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© ShodhKosh 2022. All Rights Reserved.

